Saved Bookmarks
| 1. |
7-Bromo-1,3,5-cycloheptatriene exists as an ion whereas 5-bromo-1,3-cyclopentadiene does not form an ion even in presence of Ag^+. Explain |
Answer» Solution :7-Bromo-1,3,5-cycloheptatriene, on ionization, gives tropylium ion. Since , tropylium ion contains `6 PI`-electrons which are completely delocalized, therefore, according to Huckel rule, it is AROMATIC and HENCE stable .Being highly stable , it is easily FORMED . 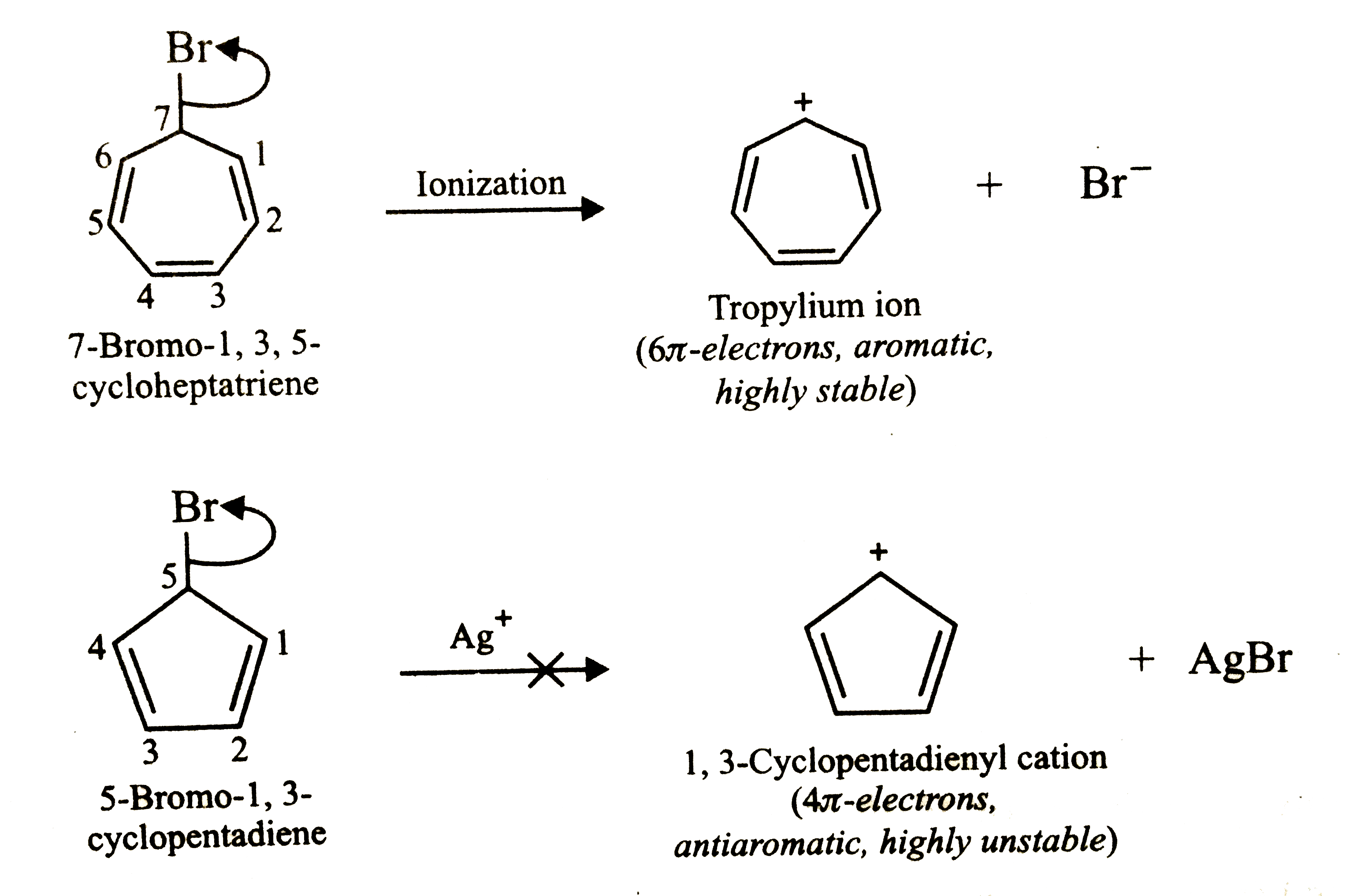 In contrast,5-bromo-1,3-cyclopentadiene, on ionization, will give 1,3-cyclopentadienyl cation which contains `4pi`-electrons and hence in antiaromatic. Being antiaromatic, it is highly unstable and hence is not formed even in the presence of `Ag^+` ion which otherwise facilitates ionization. |
|
Discussion
No Comment Found
Related InterviewSolutions
- The weight of one molecule of compound C60H122 is
- Le
- Some important compounds of sodium, notes
- find the position of Zn30 in periodic table
- How to solve ion electron method
- Calculate the amount of water produced by the combustion of 16 g of methane
- Some MCQ between chapter 1and2
- Define reciprocal proportion
- What is the spectrum of hydrogen????
- I am not able to understand ch4 piz help me