Saved Bookmarks
| 1. |
(a) Assign orientation to the three chlorotoluenes with mu = 1.3, 1.78 and 1.9 D (b) Assign orientation to the three cholornitrobenzenes with mu = 2.5, 3.4 and 4.3 D (c ) Which has higher mu . |
|
Answer» SOLUTION :`mu` of (I) GT (III) (i) Bromobenzene (II) is resonance stabillised due to delocalisation of `lp e^(-')s` of the Br ATOM in the benzene ring The `(C -B)` bond acquires some double bond character, while in (I) is a single bond, since it does not undergo resonance In other words, the `(C -Br)` bond in (II) is shorter than that in (ii) Due to more s-character `sp^(2)` hybridised `C` is more `EN` `sp^(3)` hybrid `C` atom, therefore the `sp^(2)` hybrid `C` of the `(C -Br)` bond in (II) has less tendency `C` atom of (I) As a RESULT, the `(C -Br)` bond in (I) is more polar than in (II) i e the magnitude of negative charge (`delta`-) is more on Br atom of (I) than in (II) Therefore `mu` of `(I) gt (II)` [since `mu = q xx d` (charge `xx` distance)] 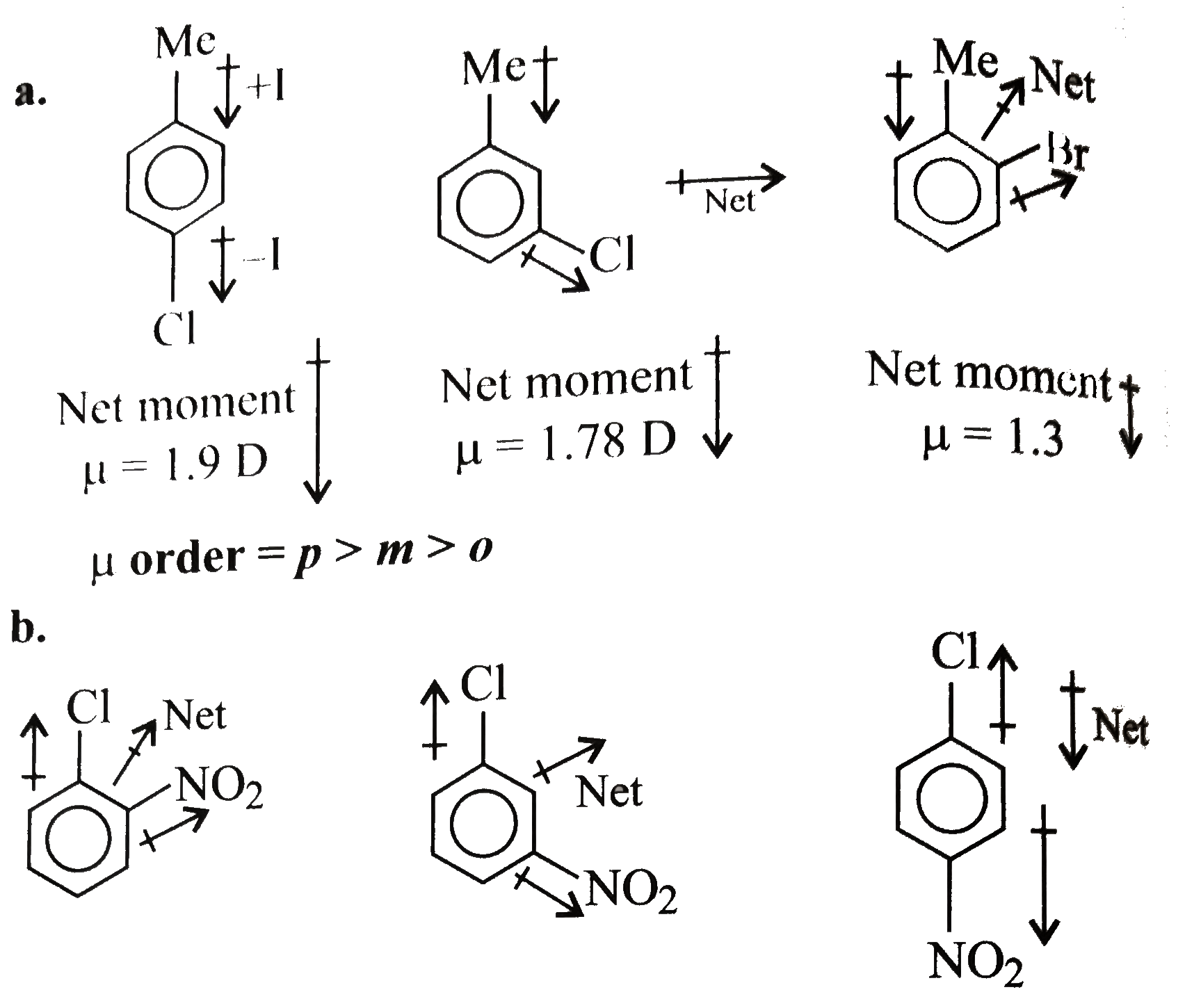 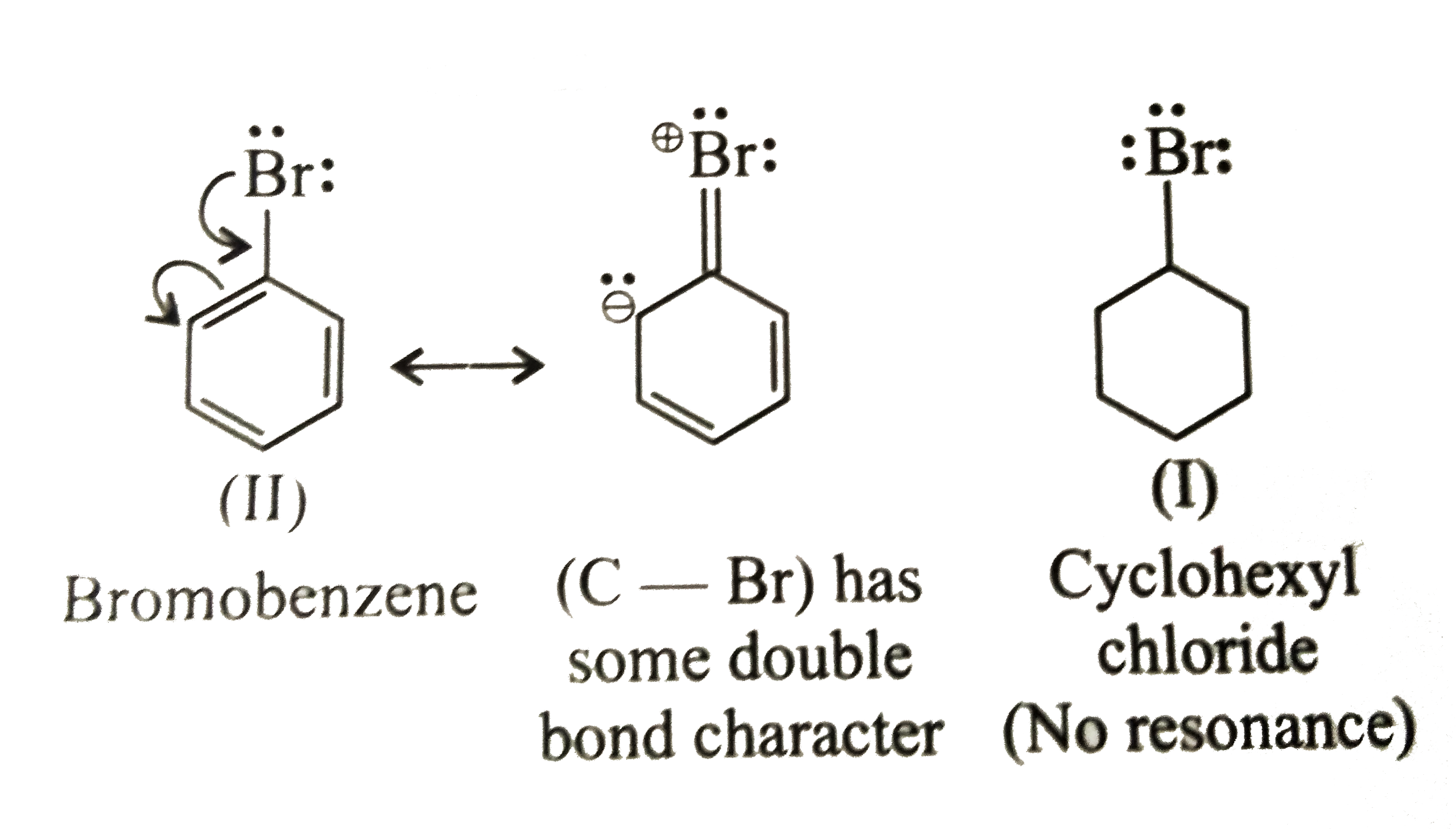 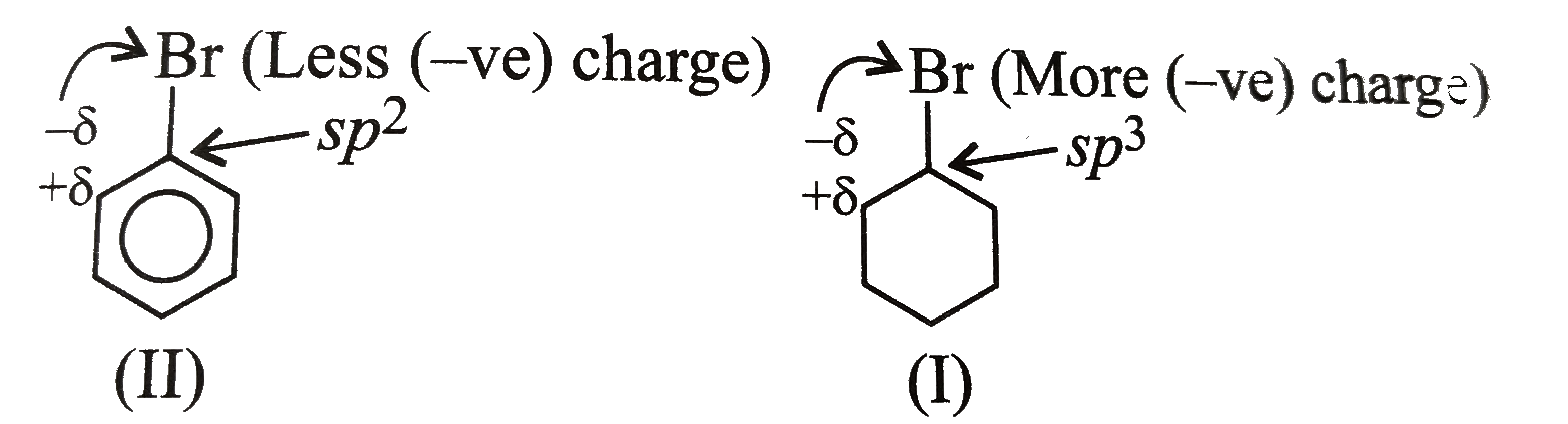
|
|
Discussion
No Comment Found
Related InterviewSolutions
- The weight of one molecule of compound C60H122 is
- Le
- Some important compounds of sodium, notes
- find the position of Zn30 in periodic table
- How to solve ion electron method
- Calculate the amount of water produced by the combustion of 16 g of methane
- Some MCQ between chapter 1and2
- Define reciprocal proportion
- What is the spectrum of hydrogen????
- I am not able to understand ch4 piz help me