Saved Bookmarks
| 1. |
a. b. c. Tetrachloroethane does not give the test for unsaturateion , i.e., it does not decolourise Br_(2) water solution. Explain why. d. e. Ricinoleic acid is isolated from castor oil. The structure of the compound is : f.Explain why 1,2-dimethyl cyclopenten or 1,2-dimethyl cyclohexen with HBr undergoes anti-( or trans-) addition but with aqueous acid undergoes both anti- and syn-( trans and cis-) additions. |
Answer» Solution :`a.`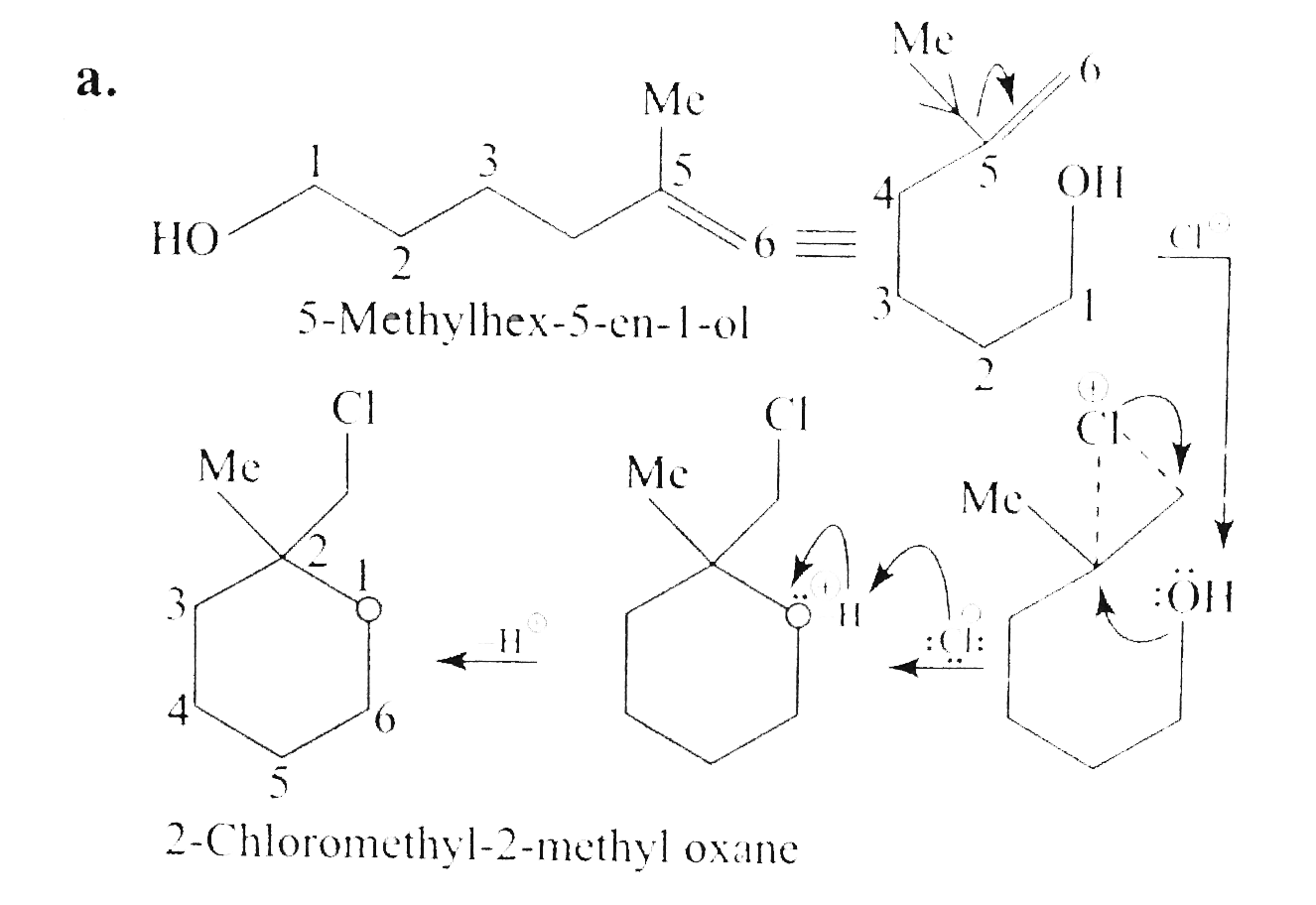 `b.` 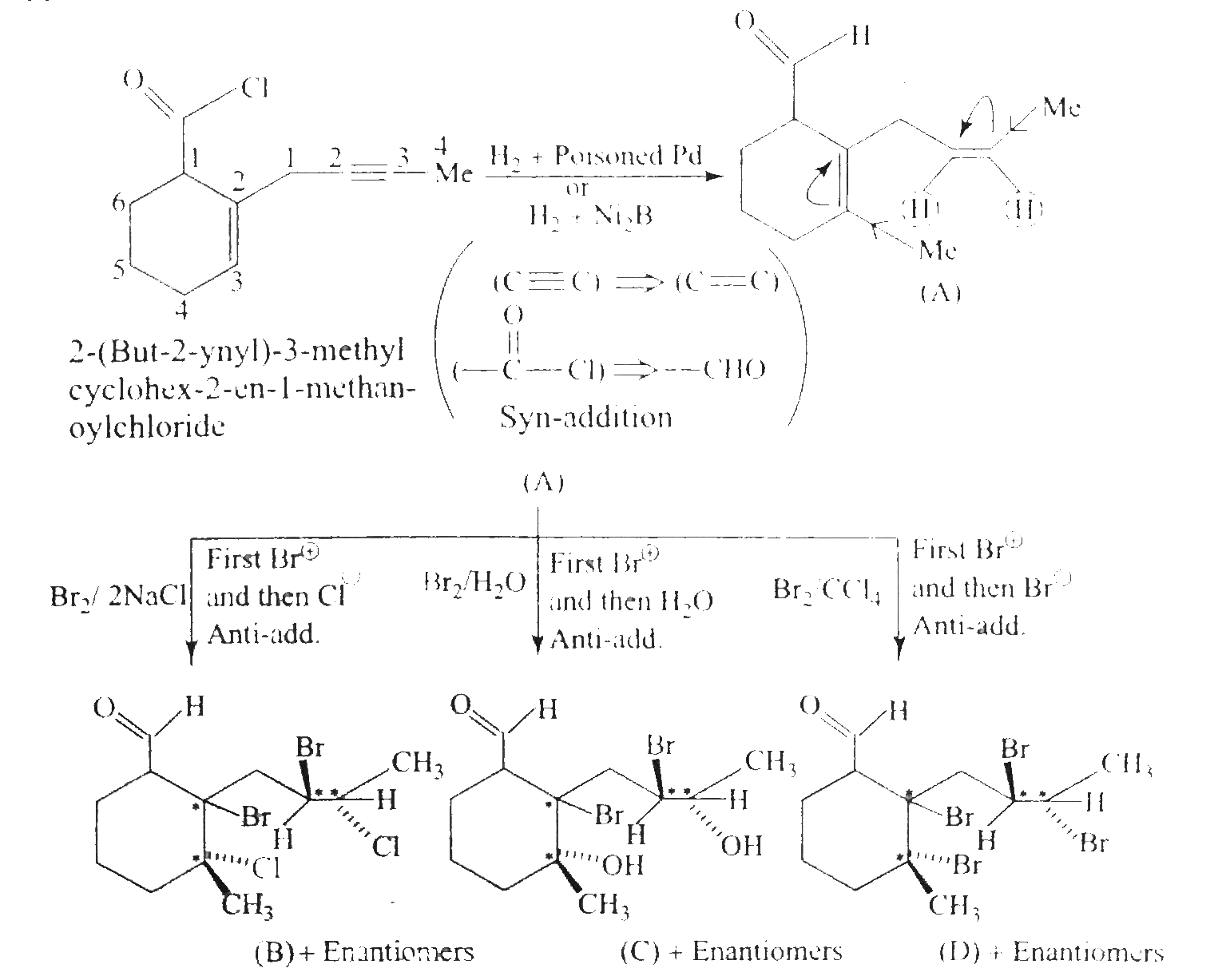 There are four chiral centrest centres and each of them shows various enentiomers also. 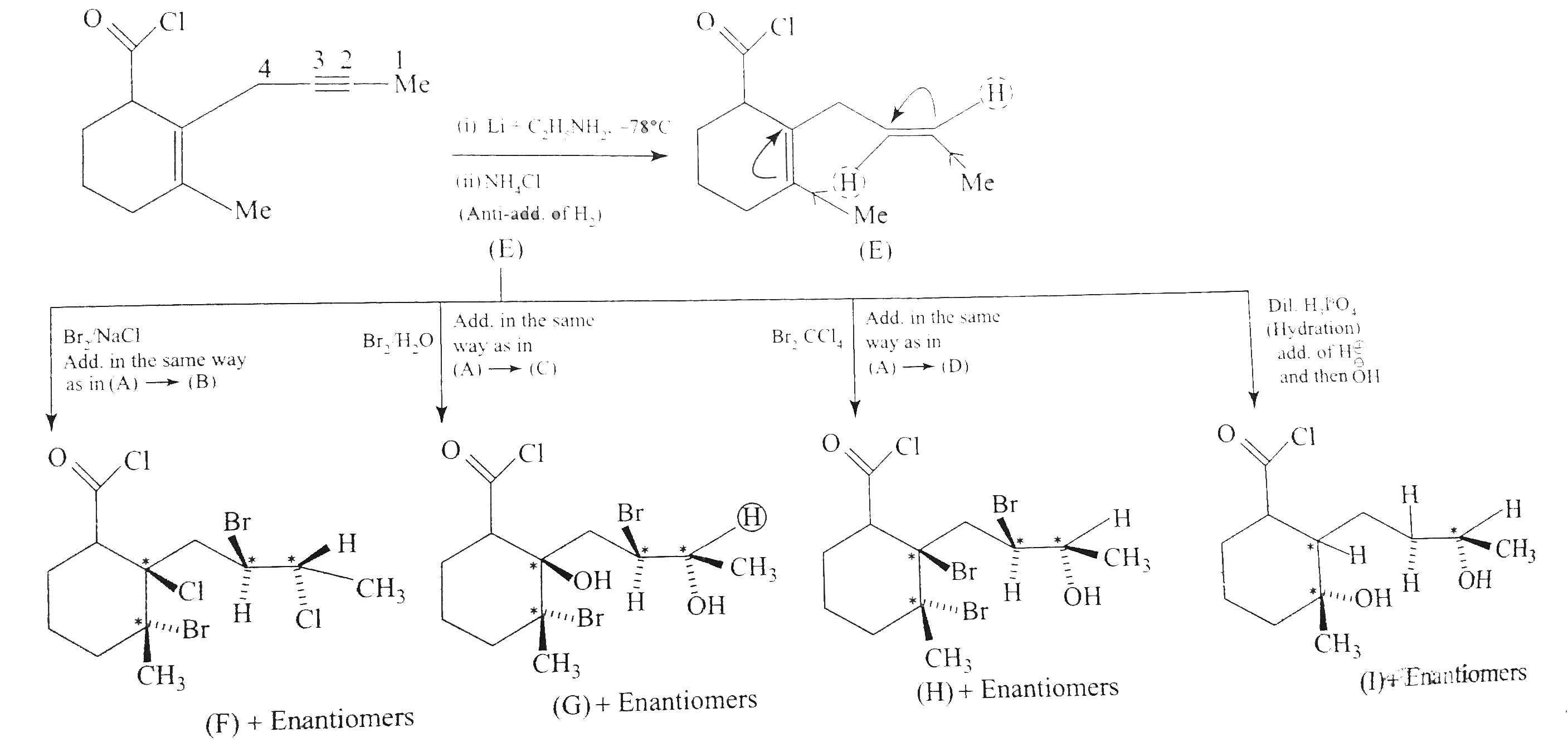 `(F), (G)` and `(H)` have four chiral centres , but `(I)` has only three chiral centres and each of them will show various enantiomers also. `c.` 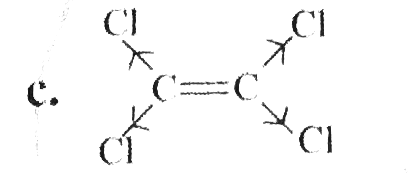 Because of the `-I` effect of four `Cl` atoms `(i.e.,` electron withdrawing nature of chlorine `)` , the electron density at the double bond is greatly reduced and an attack by electrophilic bromin `(Br^(o+))` does not occur. Hence, it does not show the test for unsaturation. `d.` 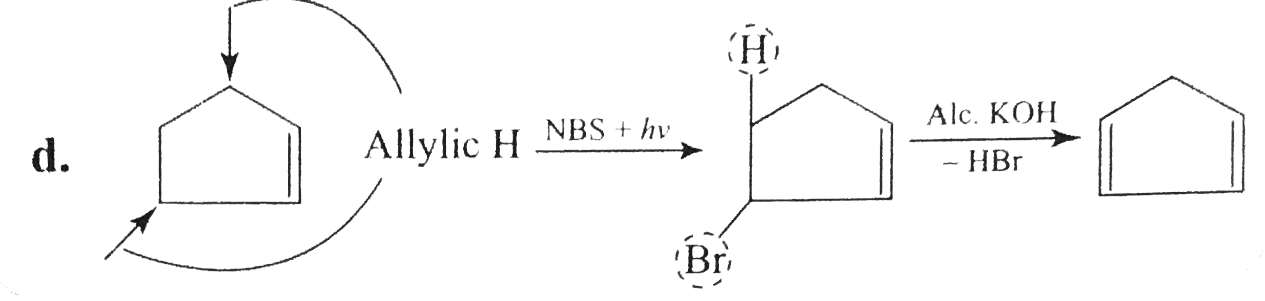 `e.` There is ONE `(C=C)` bond with different groups on each `(C=C)` bond and one chiral centre at `C=12` . It shows four geometrical land OPTICAL isomers. 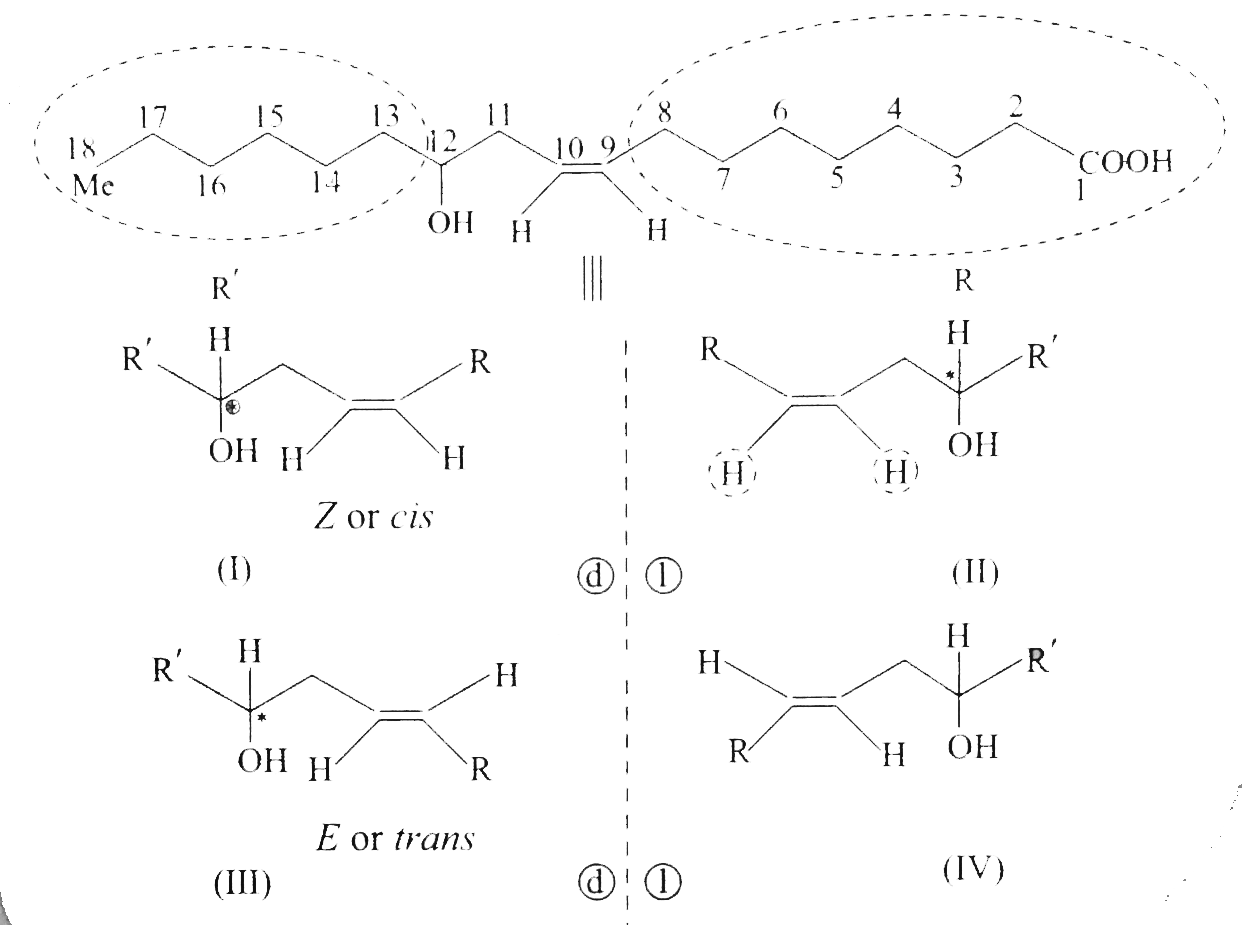 `f.` In non`-` POLAR solvents, the reaction proceeds through a briged cation such as brominimum ion and then `Br^(c-)` attacks the protonated complex from the opposite face resulting in trans `(` anti`-` addition `)`. 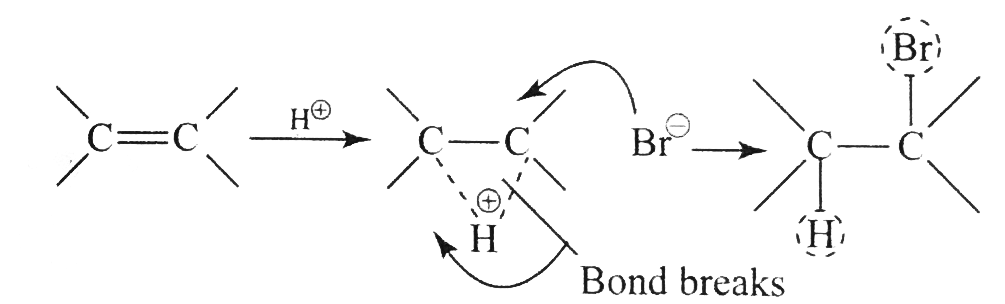 In the PRESENCE of aqueous acid, since `H_(2)O` is a good ion sovator, the protonated complex to the free `R^(o+)` that can now react with `Br^(c-)` from either face giving both syn`-` and anti `-` addition. 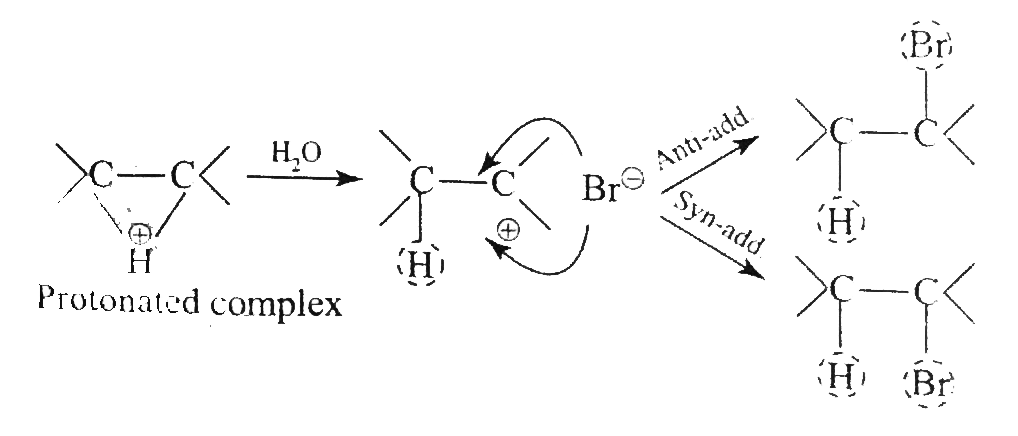 
|
|
Discussion
No Comment Found
Related InterviewSolutions
- The weight of one molecule of compound C60H122 is
- Le
- Some important compounds of sodium, notes
- find the position of Zn30 in periodic table
- How to solve ion electron method
- Calculate the amount of water produced by the combustion of 16 g of methane
- Some MCQ between chapter 1and2
- Define reciprocal proportion
- What is the spectrum of hydrogen????
- I am not able to understand ch4 piz help me