Saved Bookmarks
| 1. |
(a) [BeF_(4)]^(2-) exits, but [BeCl_(6)]^(4-) does not. Give reson. (b). Hydrated beryllium ion exists as [Be(H_(2)O)_(4)]^(2+), whereas hydrated magnesium ion exists as [Mg(H_(2)O)_(6)]^(2+). Give reason. |
Answer» Solution :a. `[BeF_(4)]^(2-)` EXISTS, but `[BeF_(6)]^(4-)` does not. This can be explained on the basis of valence ELECTRONIC configuration: 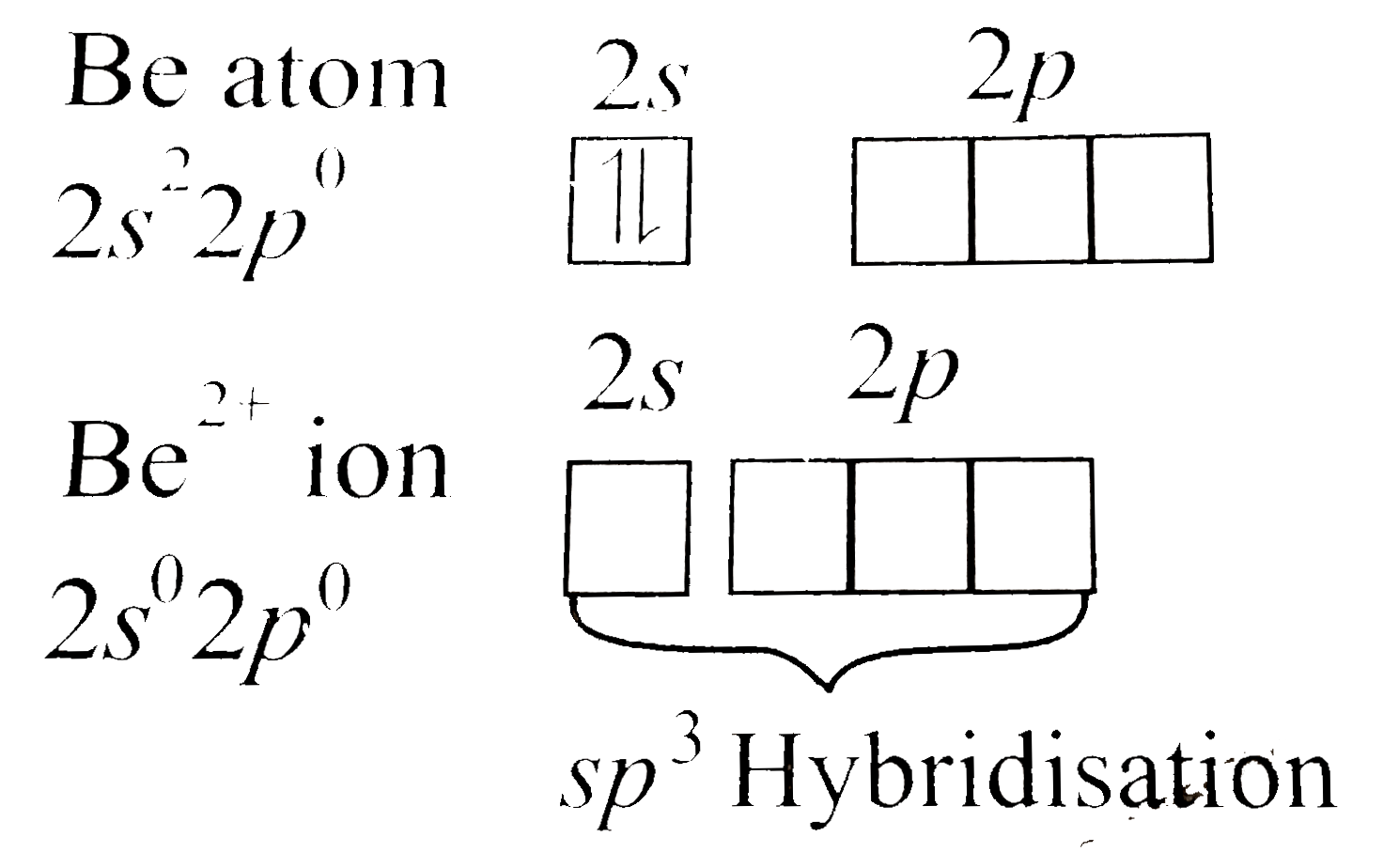 DUE to the absence of low-lying `d`-orbitals in beryllium, it cannot expand its coordination number beyond`4`, hence `[BeF_(4)]^(2-)` exists, but `[BeF_(6)]^(4-)` does not. b. Hydrated beryllium ion exists as `[Be(H_(2)O)_(4)]^(2+)`. 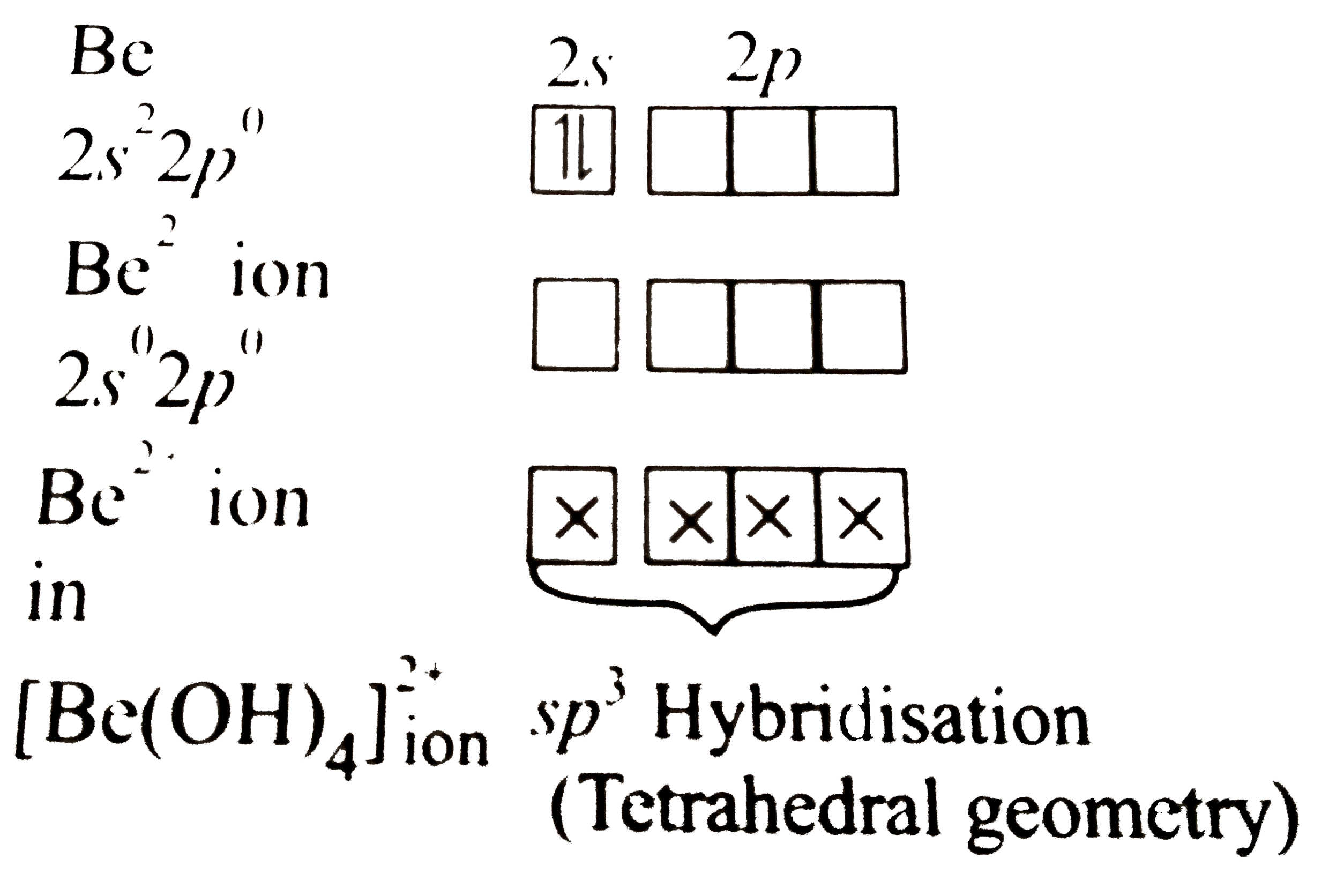 Due to the presence of only four orbitals of equivalent energy in `Be^(2+)` ion and due to the absence of low-lying `d-`orbitals in `Be^(2+)` ion, it can coordinate with four water molecules only, hence hydrated beryllium ion exists as `[Be(H_(2)O)_(4)]^(2+)`. Whereas in case of MAGNESIUM, due to the availability of low-lying d-orbitals of SUITABLE energy, `Mg` can expand its coordination number to 6 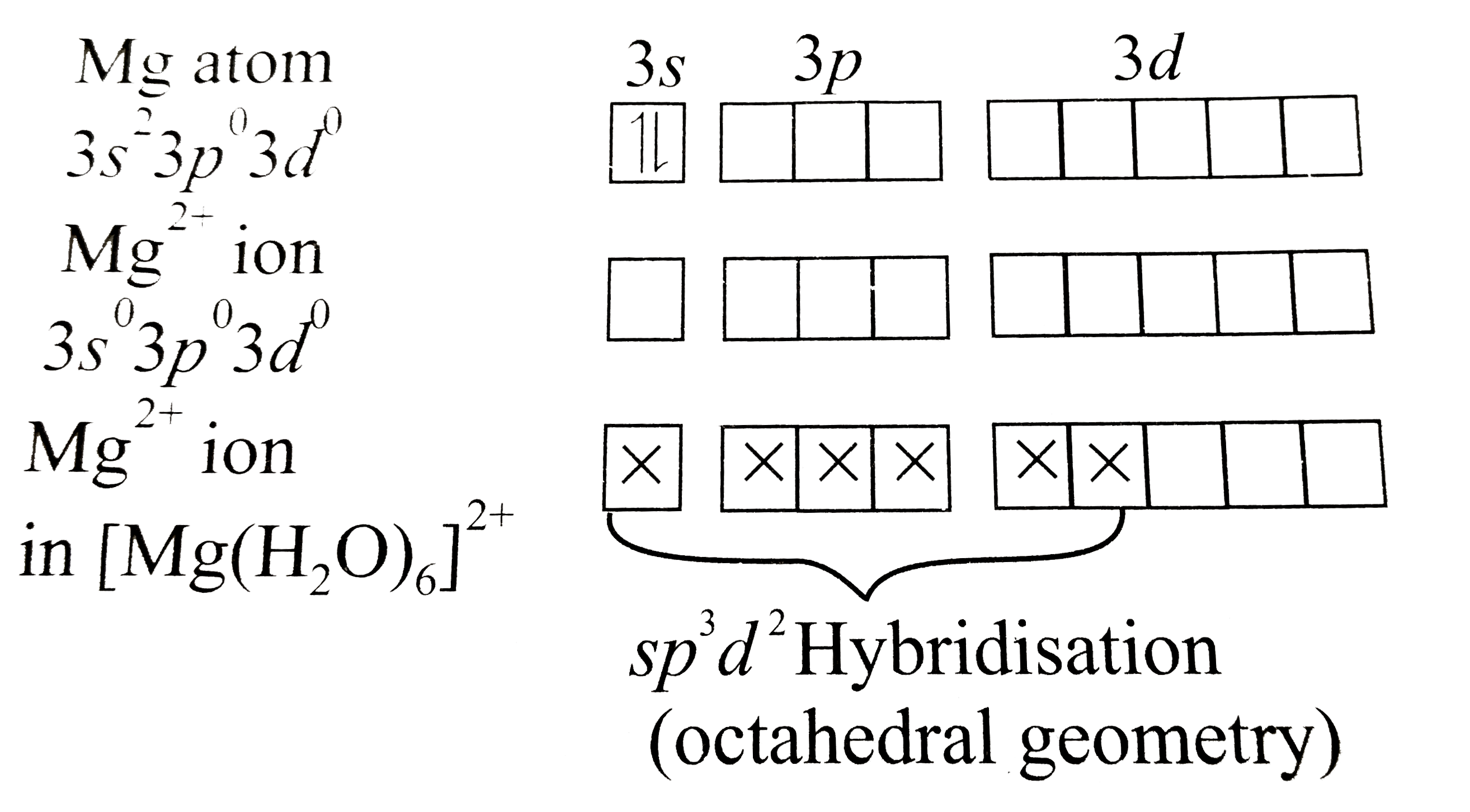 Hence, hydrated magnesium ion exists as `[Mg(H_(2)O_(6))]^(2+)` |
|
Discussion
No Comment Found
Related InterviewSolutions
- The weight of one molecule of compound C60H122 is
- Le
- Some important compounds of sodium, notes
- find the position of Zn30 in periodic table
- How to solve ion electron method
- Calculate the amount of water produced by the combustion of 16 g of methane
- Some MCQ between chapter 1and2
- Define reciprocal proportion
- What is the spectrum of hydrogen????
- I am not able to understand ch4 piz help me