Saved Bookmarks
| 1. |
A gas is compressed isothermally to half its initial volume. The same gas is compressed separately separately through an adiabatic process untial its volume is again reduced to half. Then |
|
Answer» COMPRESSING the GAS isothermally or adiabatically will REQUIRE the same AMOUNT of work. On P-V diagram, Area under adiabatic curve `gt` Area under isothermal curve. So compressing the gas through adiabatic process will require more work to bedone. 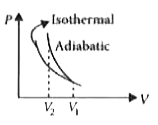
|
|
Discussion
No Comment Found
Related InterviewSolutions
- What is uniform velocity
- Derive the speed time equation by calculas method
- 9×2y - 24x^2 +16y^3.factorise?
- Give me tips for preparation of physics??
- Advantage of friction
- Derivation of equation of Newton\'s Second Law of Motion that is F=ma
- Equation of projectile?
- Unit of Permeability
- Difference between vectr and scalar
- what is centripetal force ?