Saved Bookmarks
| 1. |
(a) How is H_(2)O_(2) prepared?(b) Explain about the structure of H_(2)O_(2). |
|
Answer» Solution :(a) Hydrogen PEROXIDE can be made by adding a metal peroxide to dilute acid. `BaO_(2(s))+H_(2)SO_(4(aq))toBaSO_(4(aq))+H_(2)O_(2(aq))` (b) Structure of `H_(2)O_(2)`. 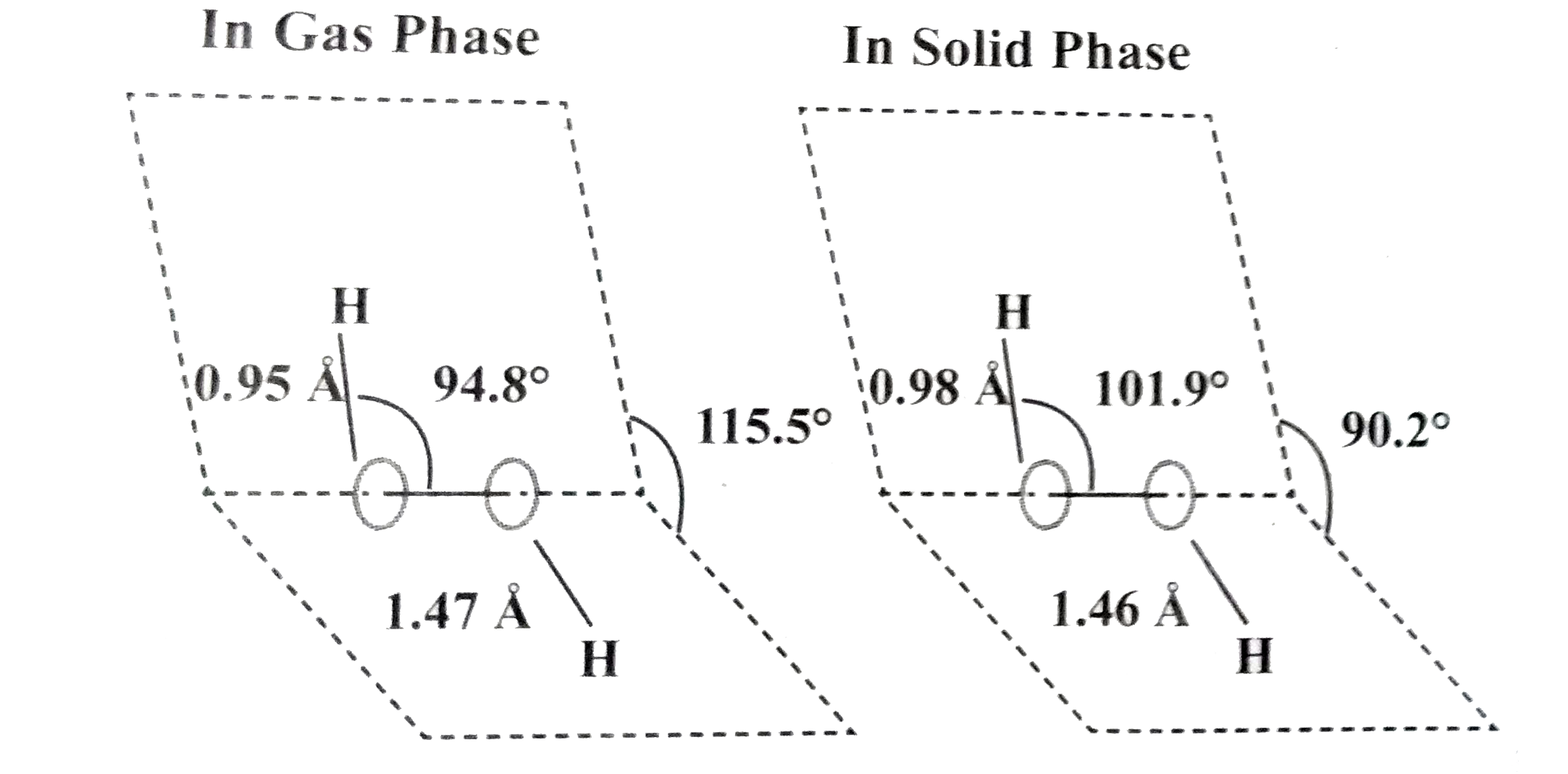 (i) `H_(2)O_(2)`has a non-polar structure. The molecular dimensions in the gas phase and solid phase differ as shown in the figure. (ii) Both in gas phase and solid phase, the `H_(2)O_(2)`, MOLECULE adopt a skew configuration due to repulsive interaction of the -OH bonds with lone pairs of electrons on each oxygen atom. (iii) Indeed, it is the smallest molecule known to show hindrance rotation about a single bond. In solid phase, the dihedral angle is SENSITIVE and hydrogen bonding decreasing from `115.5^(@)` in the gas phase to 90.2o, in the solid phase. (iv) Structurally, `H_2O_2` is represented by the dihydroxyl formula in which the TWO O-H groups do not lie in the same plane. In the solid phase of molecule, the dihedral angle reduces to 90.2" due to hydrogen bonding and the `O - O - H` angle expands from `94.8^(@)` to `101.9^(@)`. (v) One way of explaining the shape of hydrogen peroxide is that the hydrogen atoms would Tie on the pages of a partly opened book, and the oxygen atoms along the spin. |
|
Discussion
No Comment Found
Related InterviewSolutions
- The weight of one molecule of compound C60H122 is
- Le
- Some important compounds of sodium, notes
- find the position of Zn30 in periodic table
- How to solve ion electron method
- Calculate the amount of water produced by the combustion of 16 g of methane
- Some MCQ between chapter 1and2
- Define reciprocal proportion
- What is the spectrum of hydrogen????
- I am not able to understand ch4 piz help me