Saved Bookmarks
| 1. |
A hydrocarbon (A) of molecular weight 54 reacts with an excess of Br_2 in CCl_4 to give a compound (B) whose molecular weight is 593% more than that of (A). However, on catalytic hydrogenation with excess of hyrogen (A) forms (C) whose molecular weight is noly 7.4% more that that of (A). (A) reacts with CH_3CH_2Br in the presence of NaNH_2 to give another hydrocarbon (D) which on ozonolyisis yields kiketone (E). (E) on oxidation gives propionic acid. Give the structure of (A) to (E) with reason. |
|
Answer» Solution :step 1. to determine the molecular weights of COMPOUNDS (B) and (C) (i). The molecular weight of compound (A) is 54 while that of compound (B), which it GIVES on treatment with an excess of `Br_2` in `C Cl_4`, is `593%` more than that of (A). Molecular weight of `(B)=((100+593))/(100)xx54=374.22` Thus the increase in weight due to the adiiton of Br atoms is `374.22-54.0=320.22` Since atomic weight of Br is 80, the number of Br atoms added `=(320.22)/(80)=4`. As such the HYDROCARBON (A) must be an alkyne. (ii). Further since the molecular weight of compound (C) which hydrocarbon (A) gives on catalytic HYDROGENATION, is only `7.4%` more than that of (A). The molecular weight of (C) is `((100+7.4)xx54)/(100)=57.994=58`(approx) Thus, the increase in weight due to the addition of H atoms `=58-54=4`. Since the atomic weight of `H` is 1 the number of H atoms added during catalytic hydrogenation is `(4)/(1)=4`. Therefore, hyrocarbon (A) must be an alkyne. 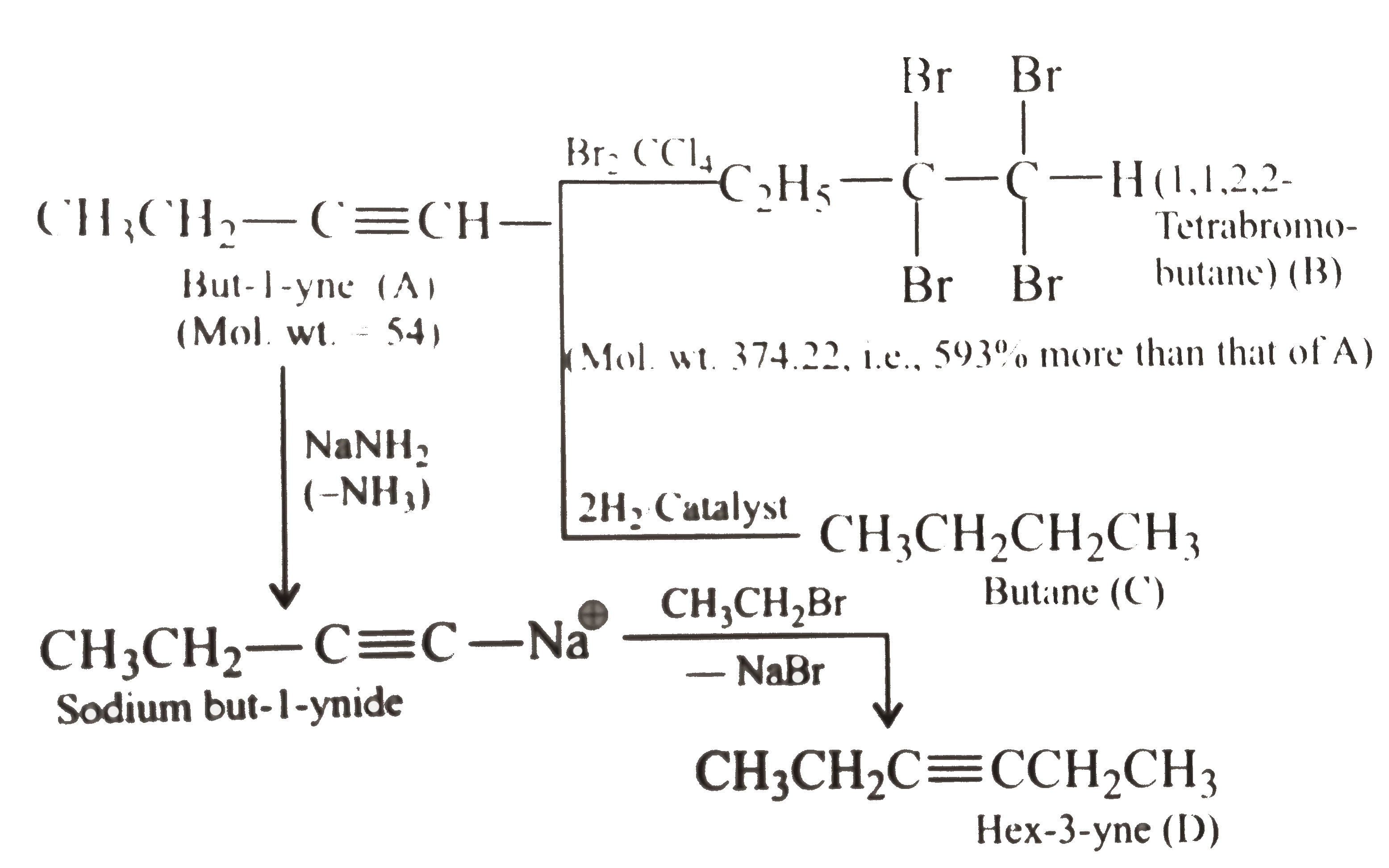 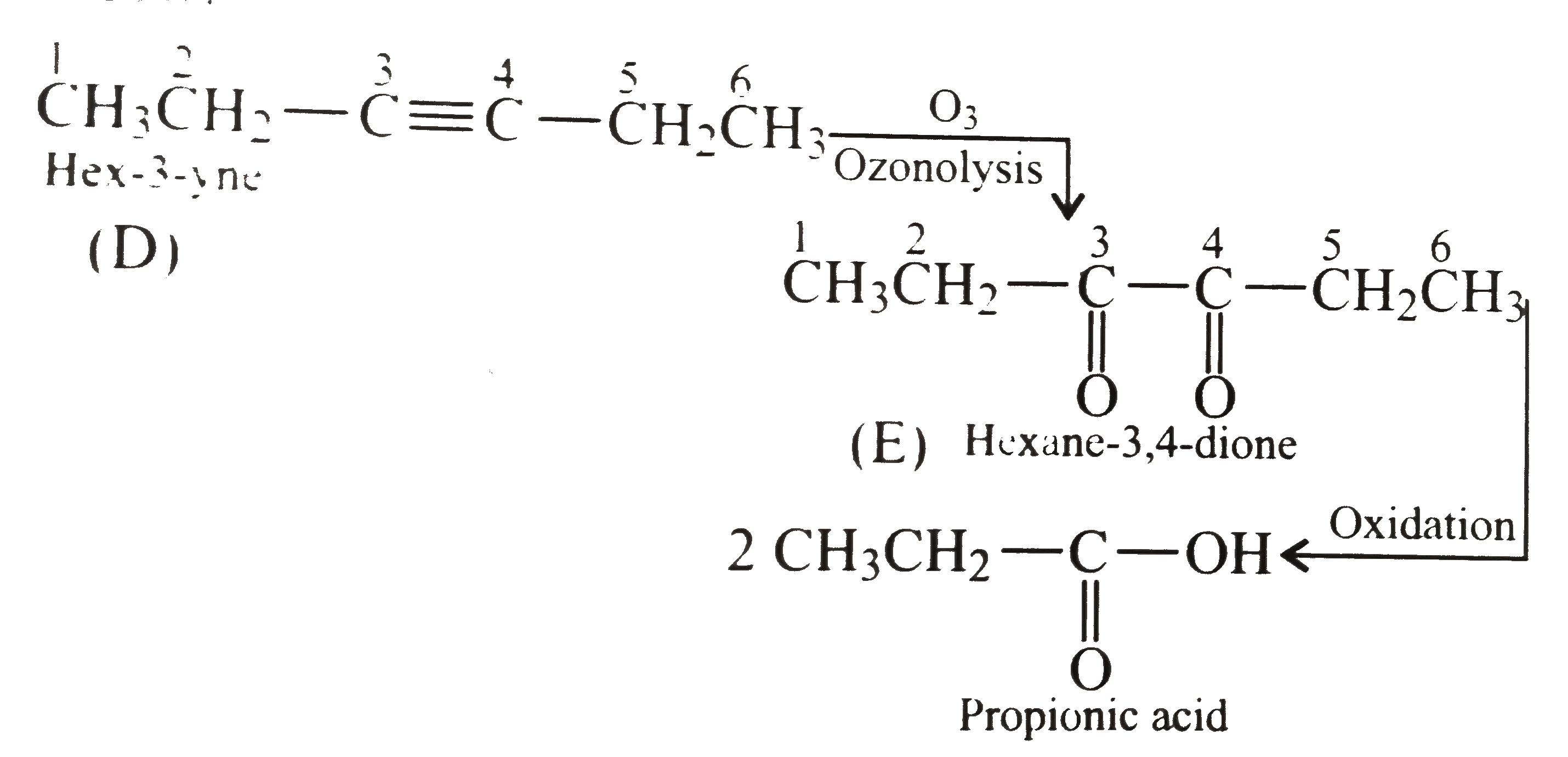
|
|
Discussion
No Comment Found
Related InterviewSolutions
- The weight of one molecule of compound C60H122 is
- Le
- Some important compounds of sodium, notes
- find the position of Zn30 in periodic table
- How to solve ion electron method
- Calculate the amount of water produced by the combustion of 16 g of methane
- Some MCQ between chapter 1and2
- Define reciprocal proportion
- What is the spectrum of hydrogen????
- I am not able to understand ch4 piz help me