Saved Bookmarks
| 1. |
A solution contains 1 g mol. Each of p-toluene diazonium chloride and p-nitrophyenl diazonium chloride. To this 1g mol. Of alkaline of phenol is added. Predict the major product. Explain you answer. |
Answer» Solution :This reaction is an example of electrophilic AROMATIC substitution. In alkaline MEDIUM, phenol generates phenoxide ION which is more electron rich than phenol and hence more reactive for electrophilic ATTACK. The electrophile ini this reaction is aryladizonium cation. Stronger the electrophile faster is the reaction. p Nitrophenyldiazonium cation is a stronger electrophile than p-toluene diazonium cation. Therefore, it couples preferntially with phenol. 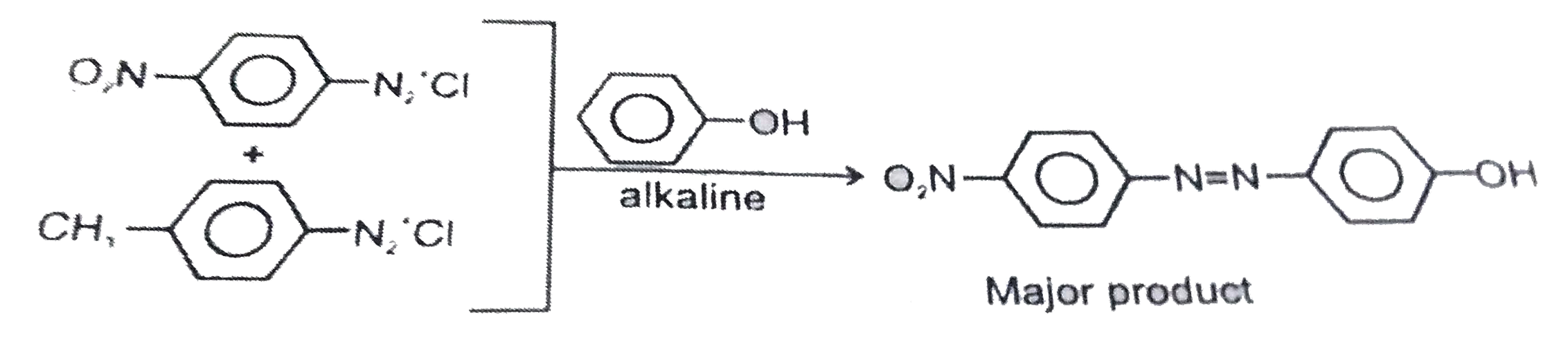
|
|
Discussion
No Comment Found
Related InterviewSolutions
- The weight of one molecule of compound C60H122 is
- Le
- Some important compounds of sodium, notes
- find the position of Zn30 in periodic table
- How to solve ion electron method
- Calculate the amount of water produced by the combustion of 16 g of methane
- Some MCQ between chapter 1and2
- Define reciprocal proportion
- What is the spectrum of hydrogen????
- I am not able to understand ch4 piz help me