Saved Bookmarks
| 1. |
A student reported the ionic radii of isocelectronic species X^(3+), Y^(2+) and Z^- as 136 pm 64 pm and 49 pm respectively. Is that order correct ? Comment. |
|
Answer» Solution :`:.` EFFECTIVE NUCLEAR charge is in the order `(Z_(EFF))_(z-)LT(Z_(eff))_X3+` and henceionic RADII should be in the order `r_z gt r_(y2+)gt r_(x3+)` `:.` The correct value are 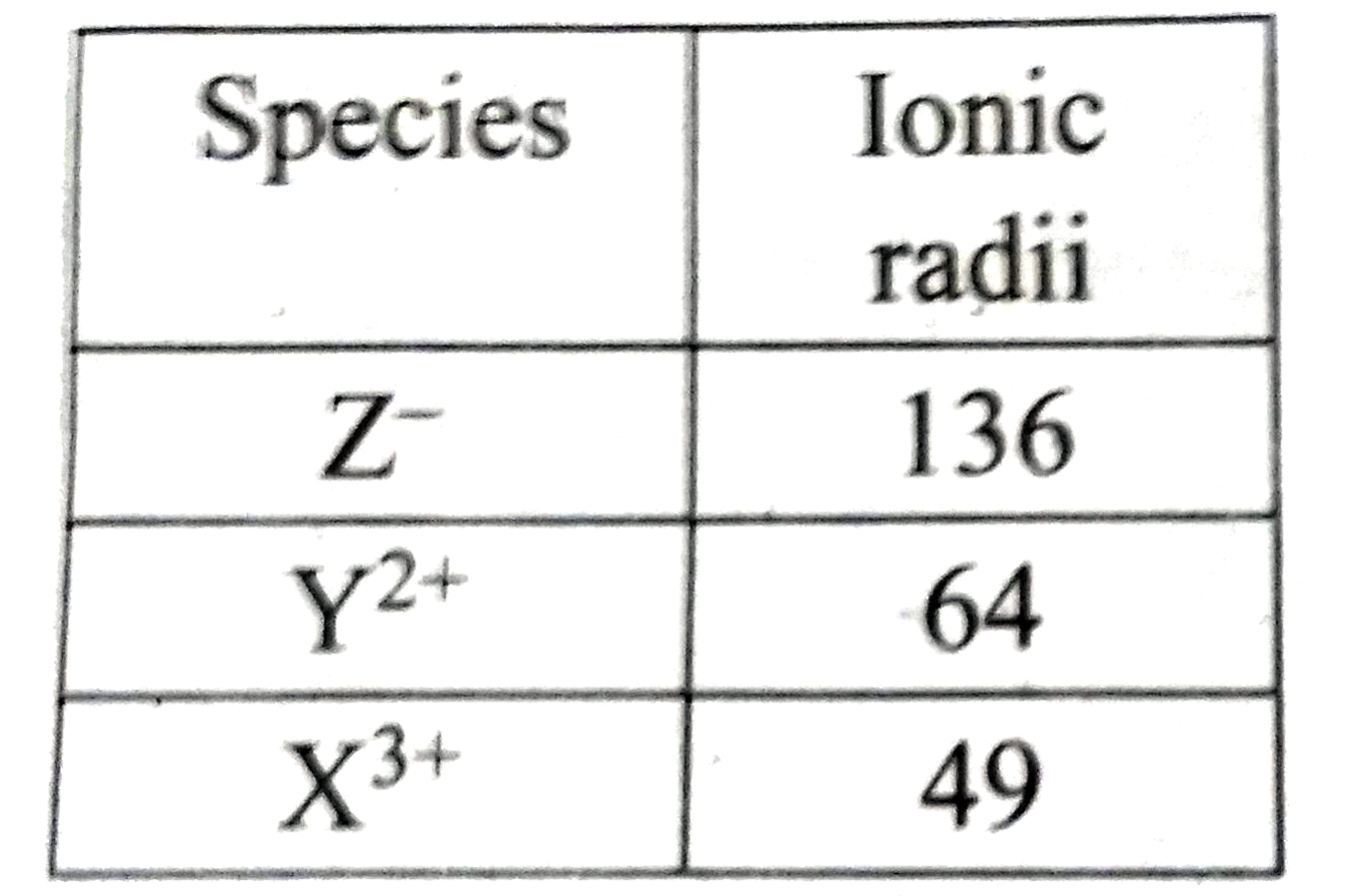
|
|
Discussion
No Comment Found
Related InterviewSolutions
- The weight of one molecule of compound C60H122 is
- Le
- Some important compounds of sodium, notes
- find the position of Zn30 in periodic table
- How to solve ion electron method
- Calculate the amount of water produced by the combustion of 16 g of methane
- Some MCQ between chapter 1and2
- Define reciprocal proportion
- What is the spectrum of hydrogen????
- I am not able to understand ch4 piz help me