Saved Bookmarks
| 1. |
Acetic acid is found to have molar mass as 120 g mol ^(-1). Prove it. |
|
Answer» SOLUTION :(i) In certain SOLVENT, solute molecules associate to from a dimer. This reduces the total number of molecules formed in solution and as a RESULT the calculated molar mass will be higher than the actual molar mass. (ii) ACETIC ACID in benzene exist as a dimer 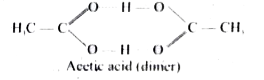 (iii) The molar mass of acetic acid calculate using colligative properties is found to be around 120 g `mol^(-1)` is two times of the actual molar mass `60 g mol ^(-1)` |
|
Discussion
No Comment Found
Related InterviewSolutions
- The weight of one molecule of compound C60H122 is
- Le
- Some important compounds of sodium, notes
- find the position of Zn30 in periodic table
- How to solve ion electron method
- Calculate the amount of water produced by the combustion of 16 g of methane
- Some MCQ between chapter 1and2
- Define reciprocal proportion
- What is the spectrum of hydrogen????
- I am not able to understand ch4 piz help me