Saved Bookmarks
| 1. |
Addition of HBr to 2-butene gives two products while that of HBr to 1-buane gives three products . Explain why ? |
Answer» SOLUTION :Addition of HBr to 2-butene gives 2-bromobutane which being a chiral molecule EXISTS as two STEREOISOMERS ( I and II) .<BR> 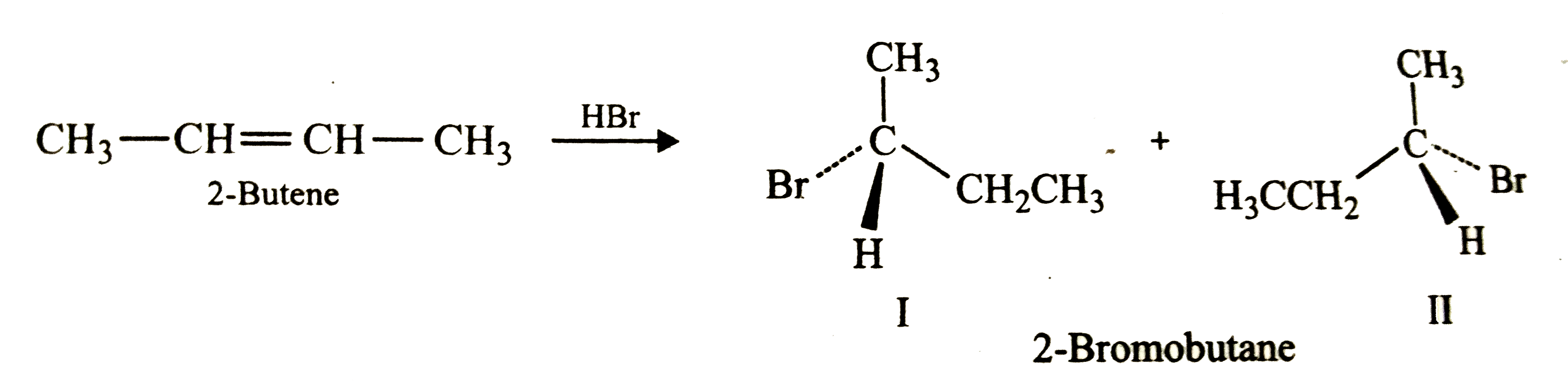 In contrast, 1-butene is an unsymmetrical molecule, therefore , it gives two products, i.e., 2-bromobutane (major product ) and 1-bromobutane (minor product). Since 2-bromobutane is a chiral molecule, it exists as two stereoisomers (I+II) as shown below above . While 1-bromobutane being a achiral molecule exists as such (III). `underset"1-Butene"(CH_3-CH_2-CH=CH_2) overset"HBr"to underset"I+II"(CH_3-CH_2-undersetunderset(Br)|overset**CH-CH_3)+underset"III"(CH_3CH_2CH_2CH_2-Br)` Thus, in all three products (I, II and III ) are FORMED. |
|
Discussion
No Comment Found
Related InterviewSolutions
- The weight of one molecule of compound C60H122 is
- Le
- Some important compounds of sodium, notes
- find the position of Zn30 in periodic table
- How to solve ion electron method
- Calculate the amount of water produced by the combustion of 16 g of methane
- Some MCQ between chapter 1and2
- Define reciprocal proportion
- What is the spectrum of hydrogen????
- I am not able to understand ch4 piz help me