Saved Bookmarks
| 1. |
Addition of HBr to 2-pentene gives |
|
Answer» 2-bromopentane only 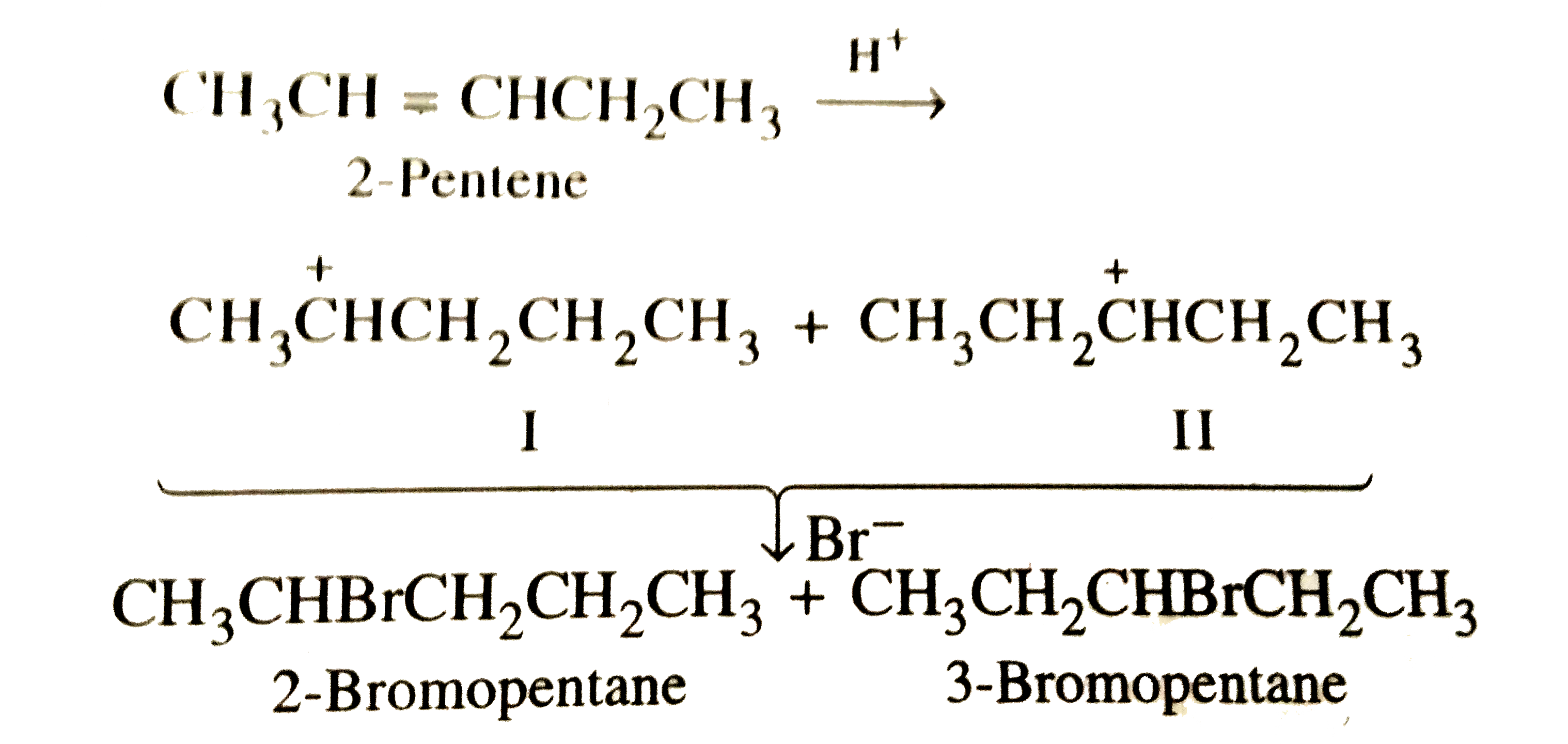 which are almost equally stable since CARBOCATION (I) is stabilized by five (3+2) HYPERCONJUGATION structures and carbocation (II) is stabilized by FOUR (2+2) hyperconjugation structures . Therefore , nucleophilic ATTACK on these gives the coresponding bromoalkanes in almost equal amounts . Of course , the yield of 2-bromopentane will be slightly more than that of 3-bromopentane. |
|
Discussion
No Comment Found
Related InterviewSolutions
- The weight of one molecule of compound C60H122 is
- Le
- Some important compounds of sodium, notes
- find the position of Zn30 in periodic table
- How to solve ion electron method
- Calculate the amount of water produced by the combustion of 16 g of methane
- Some MCQ between chapter 1and2
- Define reciprocal proportion
- What is the spectrum of hydrogen????
- I am not able to understand ch4 piz help me