Saved Bookmarks
| 1. |
Addition of HBr to propene yields 2-bromopropane, while inpresence of benzoyl peroxide, the same reaction yields 1-bromopropane. Explain and give mechanism. |
Answer» SOLUTION :Addition of HBr to propene is an ionic electrophilic addition REACTION in which the electrophile, i.e., `H^+` first ADDS to give a more stable `2^@` carbocation. In the 2nd second, the carbocation is rapidly attacked by the nucleophile `Br^-` ION to give 2-bromopropane 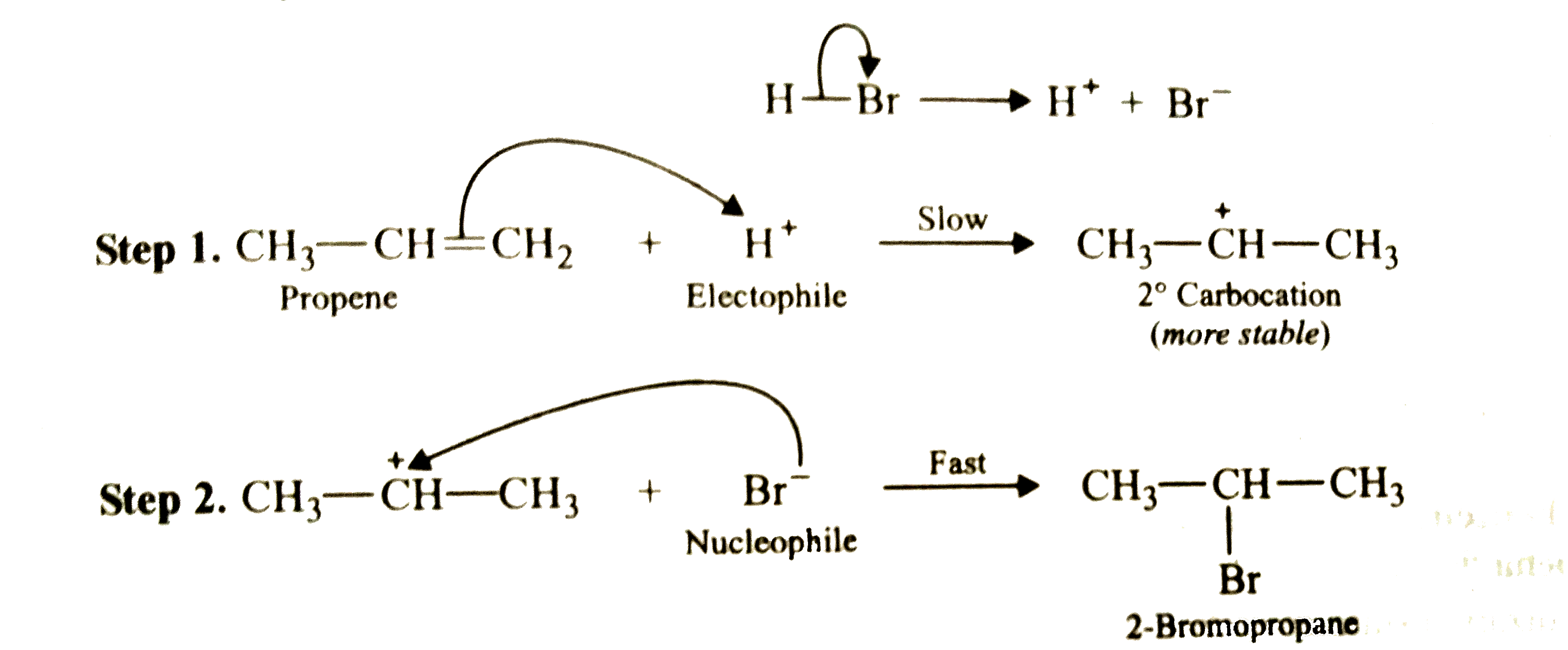 In presence of benzoyl peroxide , the reaction is still electrophilic but the electrophile here is a `oversetdot(Br)` free radical which is obtained by the action of benzoyl peroxide on HBr 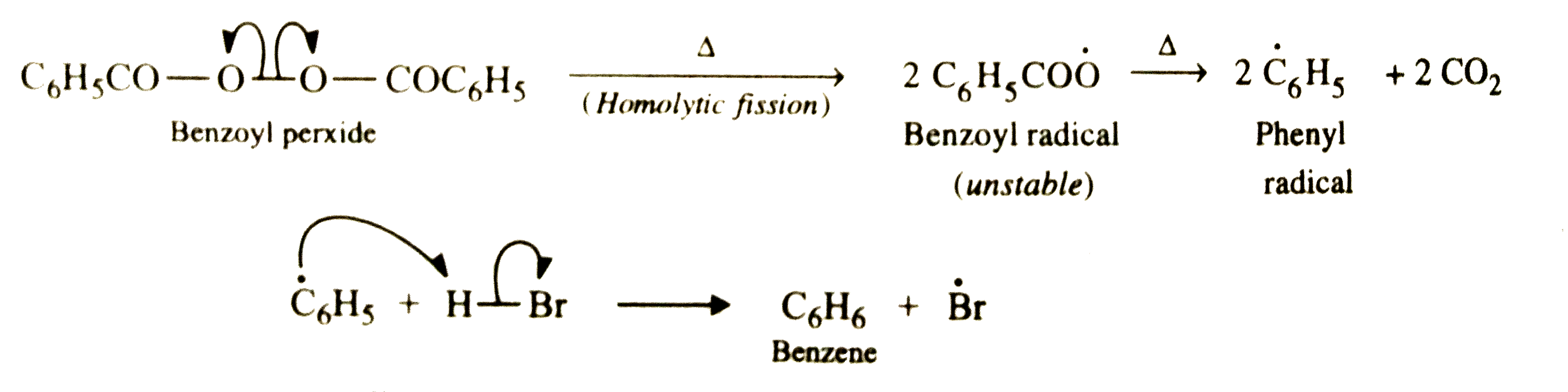 In the first step, `oversetdot(Br)` radical adds to propene in such a way as to generate a more stable `2^@` radical . In the second step, the free radical thus obtained rapidly abstracts a hydrogen atom from HBr to give 1-bromopropane. 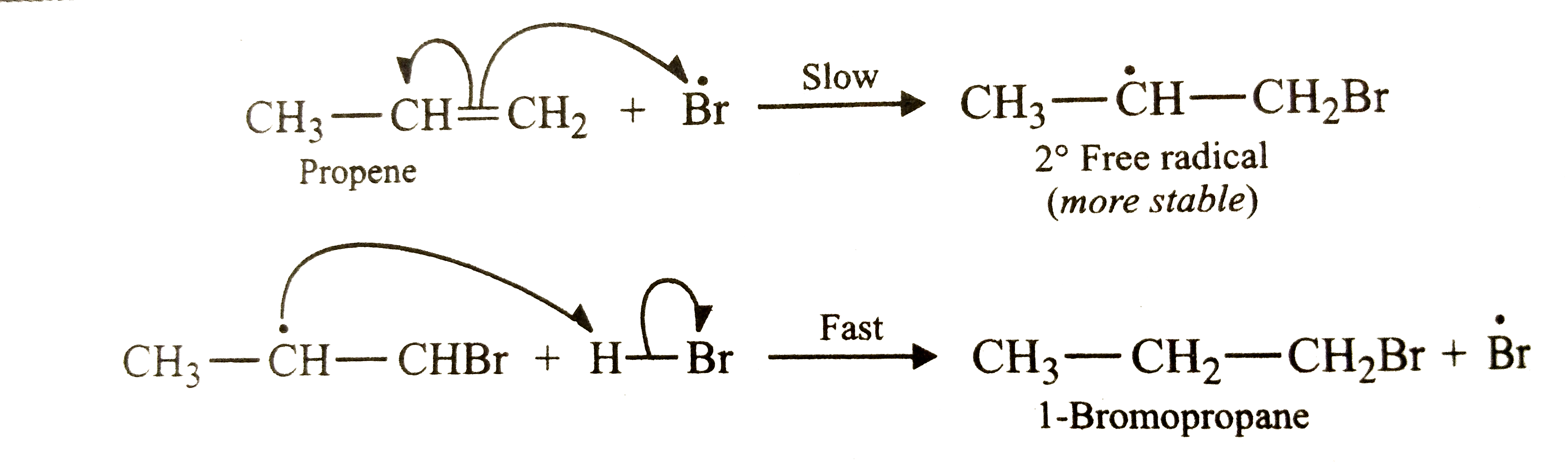 it is evident that although both reactions (i.e., in presence or absence of benzoyl peroxide ) are electrophilic addition but it is due to different order or sequence of addition of H and Br atoms which gives different PRODUCTS. |
|
Discussion
No Comment Found
Related InterviewSolutions
- The weight of one molecule of compound C60H122 is
- Le
- Some important compounds of sodium, notes
- find the position of Zn30 in periodic table
- How to solve ion electron method
- Calculate the amount of water produced by the combustion of 16 g of methane
- Some MCQ between chapter 1and2
- Define reciprocal proportion
- What is the spectrum of hydrogen????
- I am not able to understand ch4 piz help me