Saved Bookmarks
| 1. |
Alk aline earth metal (A), belongs to 3rd period reacts with oxygen and nitrogen to form compound (B) and (C) respectively. It undergo metal displacement reaction with AgNO_3 solution to form compound (D). |
|
Answer» Solution :(i) An alkaline earth (A) metal belongs to third period is magnesium (Mg) (ii) Magnesium REACTS with oxygen to form magnesium oxide (MgO) (B) `2Mg +O_(2)tounderset(Magnesium oxide)(2MgO_(2))` (iii) Magnesium reacts with NITROGEN to form magnesium nitride `Mg_(3)N_(2)(C)` `3Mg+N_(2)O_(2)tounderset("Magnesium nitride")(Mg_(3)O)` (iv) Magnesium UNDERGOES metal displacement reaction with `AgNO_(3)` solution to form magnesium nitrate `Mg(NO_(3))_(2) (D)` `Mg+2AgNO_(3) tounderset("Magnesium nitrate") (Mg(NO_(3))_(2) +)2AG` 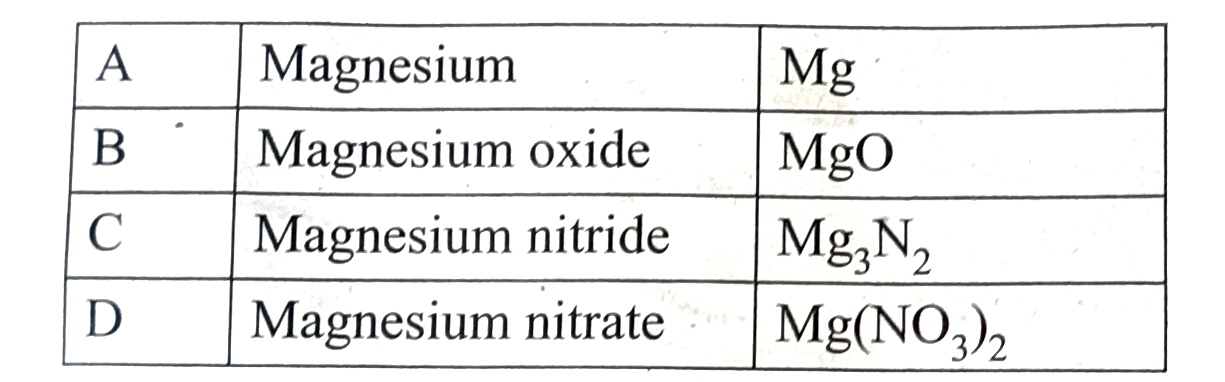
|
|
Discussion
No Comment Found
Related InterviewSolutions
- The weight of one molecule of compound C60H122 is
- Le
- Some important compounds of sodium, notes
- find the position of Zn30 in periodic table
- How to solve ion electron method
- Calculate the amount of water produced by the combustion of 16 g of methane
- Some MCQ between chapter 1and2
- Define reciprocal proportion
- What is the spectrum of hydrogen????
- I am not able to understand ch4 piz help me