Saved Bookmarks
| 1. |
Although bothCO_(2) and H_(2) Oare triatomic molecules, the shape of H_(2)Omolecule is bent while that ofCO_(2) is linear . Explain these on the basis of dipole mement. |
|
Answer» Solution :The dipole mement STUDIES show that the dipole moment of `CO_(2)`molecule is zero . This is POSSIBLE only if ` CO_(2)`is a linear molecule ` ((O = C=O))` so that diple moment of C-Oare equal and opposite and HENCE cancel out . On the other hand, `H_(2) O ` molecule is found to have a net dipole moment (1.84 D) thought it contains 2 O-H bonds . This shows that it is a bent molecule 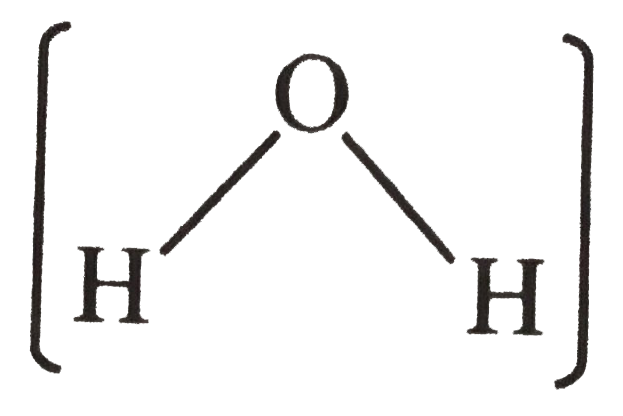
|
|
Discussion
No Comment Found
Related InterviewSolutions
- The weight of one molecule of compound C60H122 is
- Le
- Some important compounds of sodium, notes
- find the position of Zn30 in periodic table
- How to solve ion electron method
- Calculate the amount of water produced by the combustion of 16 g of methane
- Some MCQ between chapter 1and2
- Define reciprocal proportion
- What is the spectrum of hydrogen????
- I am not able to understand ch4 piz help me