Saved Bookmarks
| 1. |
Although C-D bond is stronger than C-H bond, yet (CH_(3))_(3)C^(+)(i) is more stable than (CD_(3))_(3)C^(+) (ii) Why so ? |
Answer» Solution :Both carbocations (i) and (ii) are stabilized by hyperconjugation as shown below: 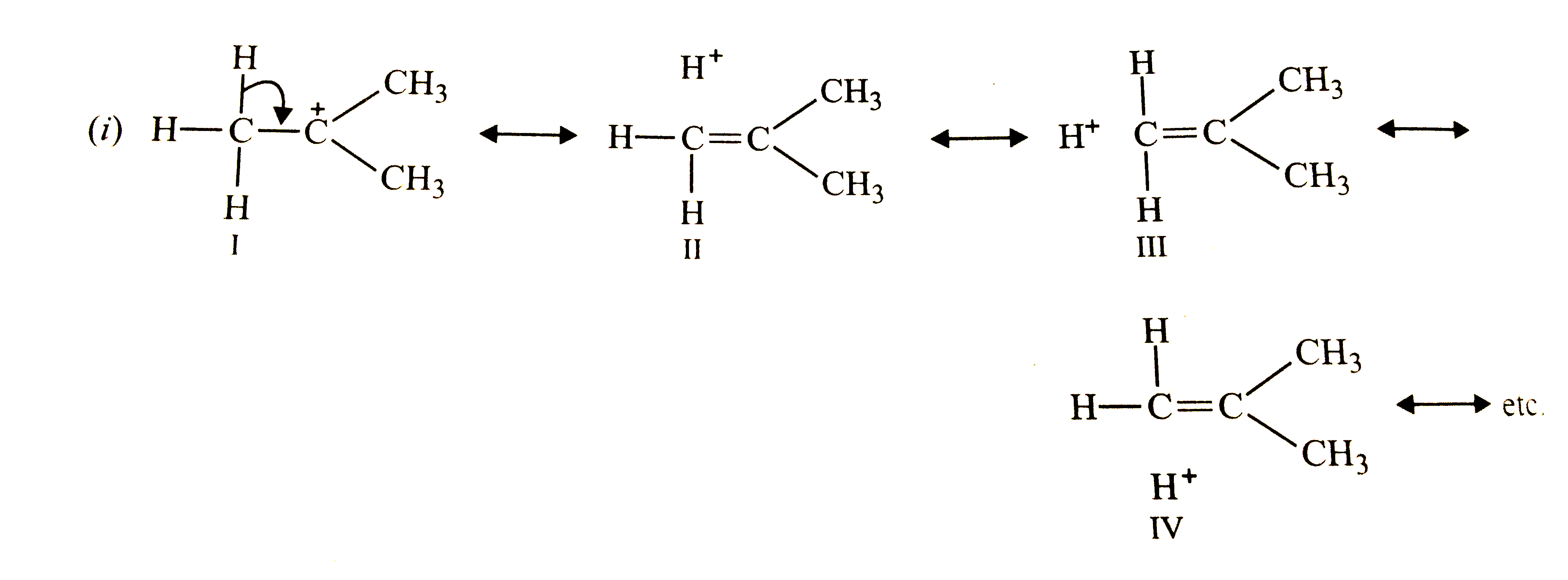 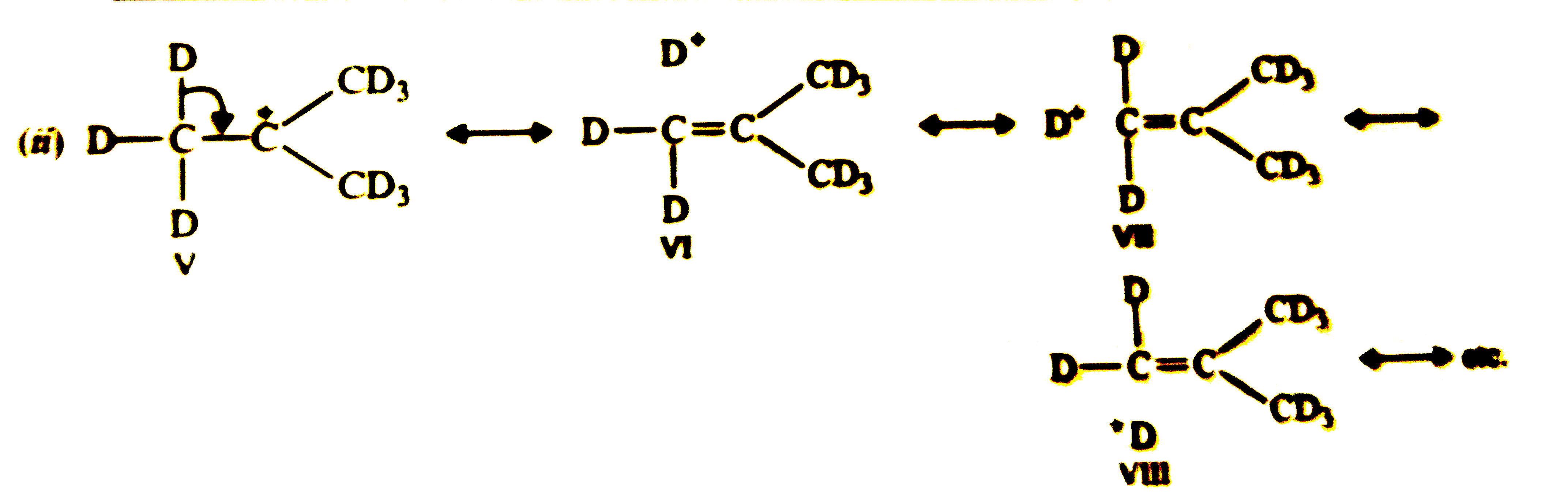 Due to stronger C-D bond, CONTRIBUTION of STRUCTURES (V-VIII) towards stability of carbocations, `(CD_(3))_(3)C^(+)` is less than those of structures (I-IV) for carbocation, `(CH_(3))_(3)C^(+)`, therefore, carbocation (i) is more stable than carbocation (ii). This effec is also sometimes called as `beta-` or secondary isotope EFFECT. |
|
Discussion
No Comment Found
Related InterviewSolutions
- The weight of one molecule of compound C60H122 is
- Le
- Some important compounds of sodium, notes
- find the position of Zn30 in periodic table
- How to solve ion electron method
- Calculate the amount of water produced by the combustion of 16 g of methane
- Some MCQ between chapter 1and2
- Define reciprocal proportion
- What is the spectrum of hydrogen????
- I am not able to understand ch4 piz help me