Saved Bookmarks
| 1. |
Although carbocations are alwaysplanar but carbanions and free radicals can assume either planar or pyramidal geometry. Why is it so ? Explain. |
Answer» Solution :The simple alkyl carbanions are pyramidal. For example, 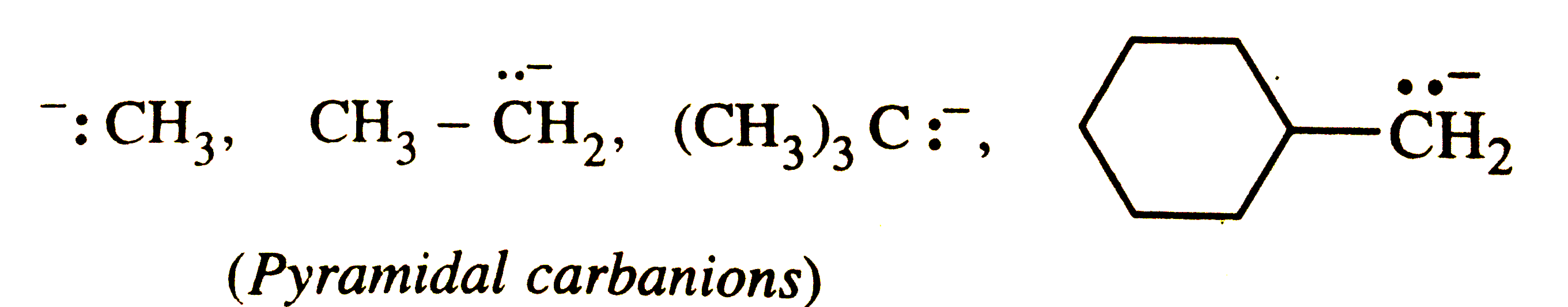 However, carbanions in which the C-atom carrying the NEGATIVE charge is adjacent to a double bond or a benzene ring, are planar due to stabilization by RESONANCE. Thus, allyl and benzyl carbanions are planar. 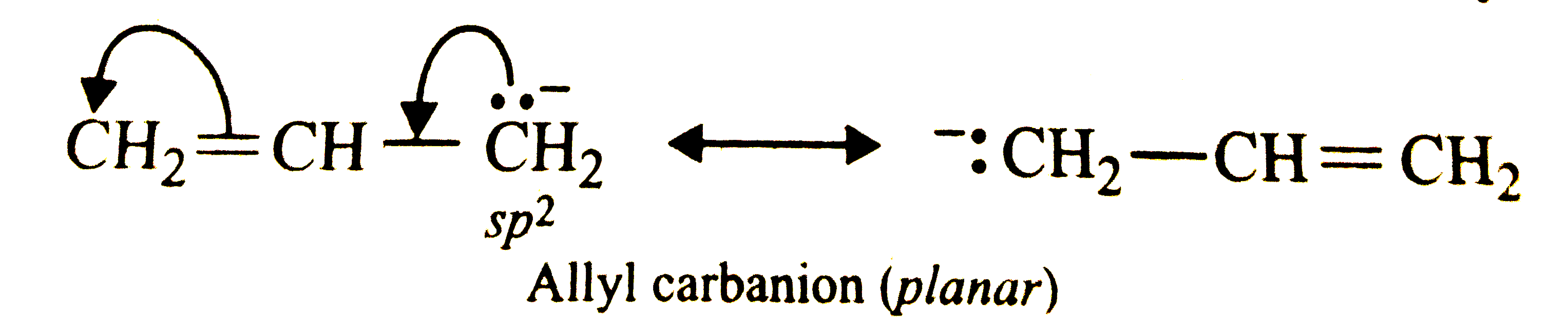 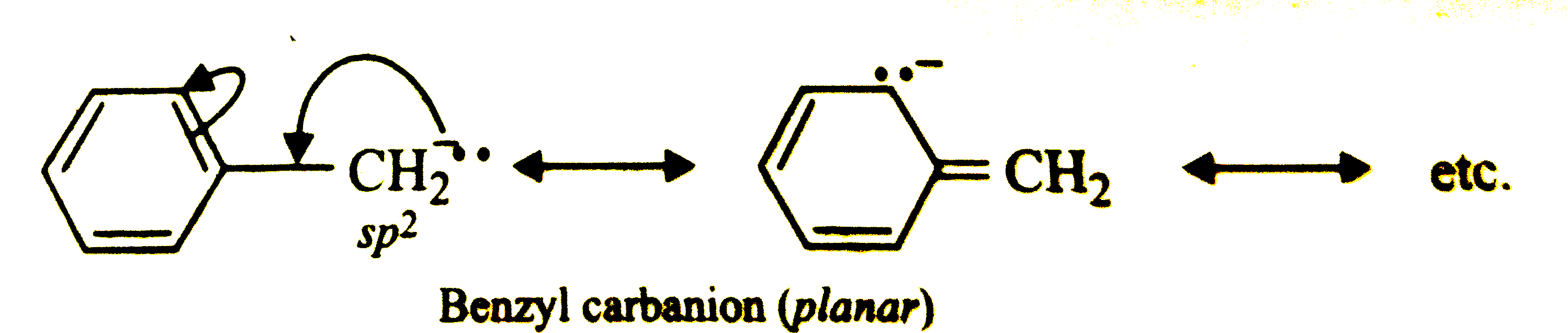 Most of the free radicals are planar but free radicals in which the carbon atom carrying the ODD ELECTRON is connected to a bridge head carbon or highly electronegative element are pyramidal. For examples. `CF_(3)` has pyramidal shape. |
|
Discussion
No Comment Found
Related InterviewSolutions
- The weight of one molecule of compound C60H122 is
- Le
- Some important compounds of sodium, notes
- find the position of Zn30 in periodic table
- How to solve ion electron method
- Calculate the amount of water produced by the combustion of 16 g of methane
- Some MCQ between chapter 1and2
- Define reciprocal proportion
- What is the spectrum of hydrogen????
- I am not able to understand ch4 piz help me