Saved Bookmarks
| 1. |
Among NH_(3), H_(2)O and HF , which would you expect to have highest magnitude of hydrogen bonding and why ? |
Answer» Solution :Due to GREATER electronegativity of N, O and F over, H, all these molecules undergo intermolecular H-bonding 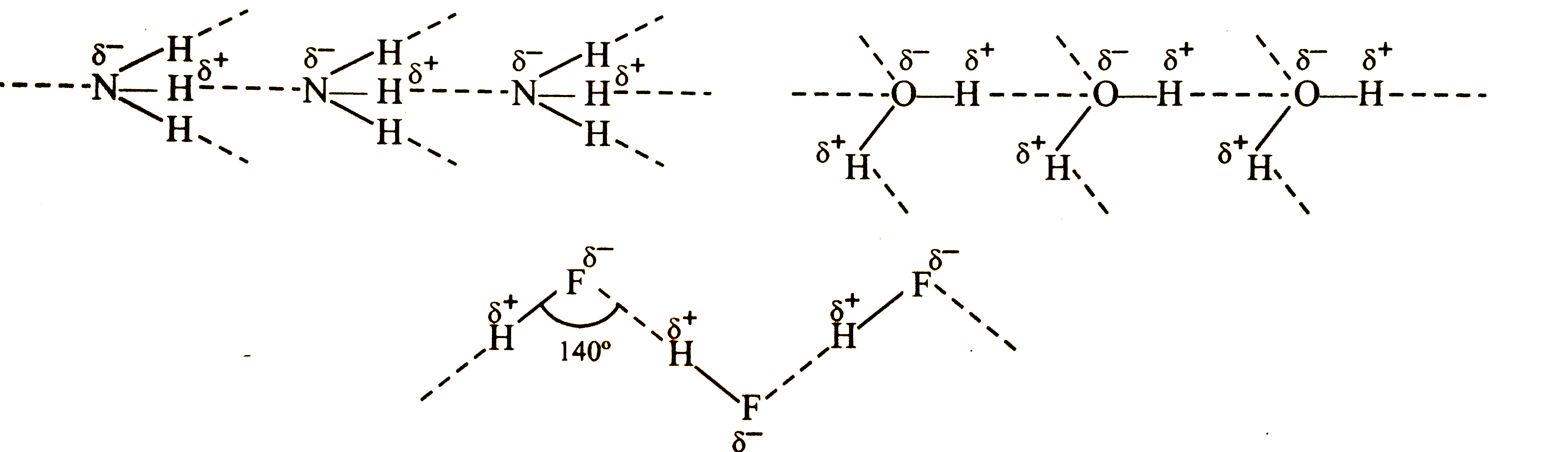 However,since electronegativity of F is the highest, THEREFORE, magnitude of the +ve charge on HYDORGEN and -ve charge of F is the highest and HENCE electrostatic attraction or the H-bonding is the strongest in H-F |
|
Discussion
No Comment Found
Related InterviewSolutions
- The weight of one molecule of compound C60H122 is
- Le
- Some important compounds of sodium, notes
- find the position of Zn30 in periodic table
- How to solve ion electron method
- Calculate the amount of water produced by the combustion of 16 g of methane
- Some MCQ between chapter 1and2
- Define reciprocal proportion
- What is the spectrum of hydrogen????
- I am not able to understand ch4 piz help me