Saved Bookmarks
| 1. |
Figure below shows two paths that may be taken by a gas to go from state A to a state C. In process AB, 400 Jof heat is added to the system and in proces BC, 100 J of heat isadded to the system. The heat absorbed by the system in the process AC will be |
|
Answer» Solution :As initial and final points are same so `Delta U_(ABC) = Delta U_(AC)` AB is isochoric PROCESS. `Delta W_(AB) = 0` `Delta Q_(AB) = Delta U_(AB) = 400 J` B is isobaric process. `Delta Q_(BC) = Delta U_(BC) + Delta W_(BC)` `100 = Delta U_(BC) + 6 xx 10^(4)` `(4 xx 10^(-3) - 2 xx 10^(-3))` `100 = Delta U_(BC) + 12 xx 10` `Delta U_(BC) = 100 - 120 = -20 J` As, `Delta U_(ABC) = Delta U_(AC)` `Delta U_(AB) + Delta U_(BC) = Delta Q_(AC) - Delta W_(AC)` `400 - 20 = Delta Q_(AC) - (2 xx 10^(4) xx 2 xx 10^(-3) + (1)/(2) xx 2 xx 10^(-3) xx 4 xx 10^(4))` `380 = Delta Q_(AC) - (40 + 40), Delta Q_(AC) = 380 + 80 = 460 J` 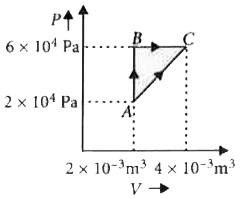
|
|
Discussion
No Comment Found
Related InterviewSolutions
- What is uniform velocity
- Derive the speed time equation by calculas method
- 9×2y - 24x^2 +16y^3.factorise?
- Give me tips for preparation of physics??
- Advantage of friction
- Derivation of equation of Newton\'s Second Law of Motion that is F=ma
- Equation of projectile?
- Unit of Permeability
- Difference between vectr and scalar
- what is centripetal force ?