InterviewSolution
Saved Bookmarks
| 1. |
Lattice energy of Na_(2)CO_(3) is -205 kJ/mole and hydration energy of Na^(+) ion & CO_(3)^(2-) ion are -80kJ/mole and -40kJ/mole respectively. What can be predicted about solubility of Na_(2)CO_(3) in water from the above data. |
|
Answer» The SOLUBILITY of `Na_(2)CO_(3)` will increase will increase in temperature 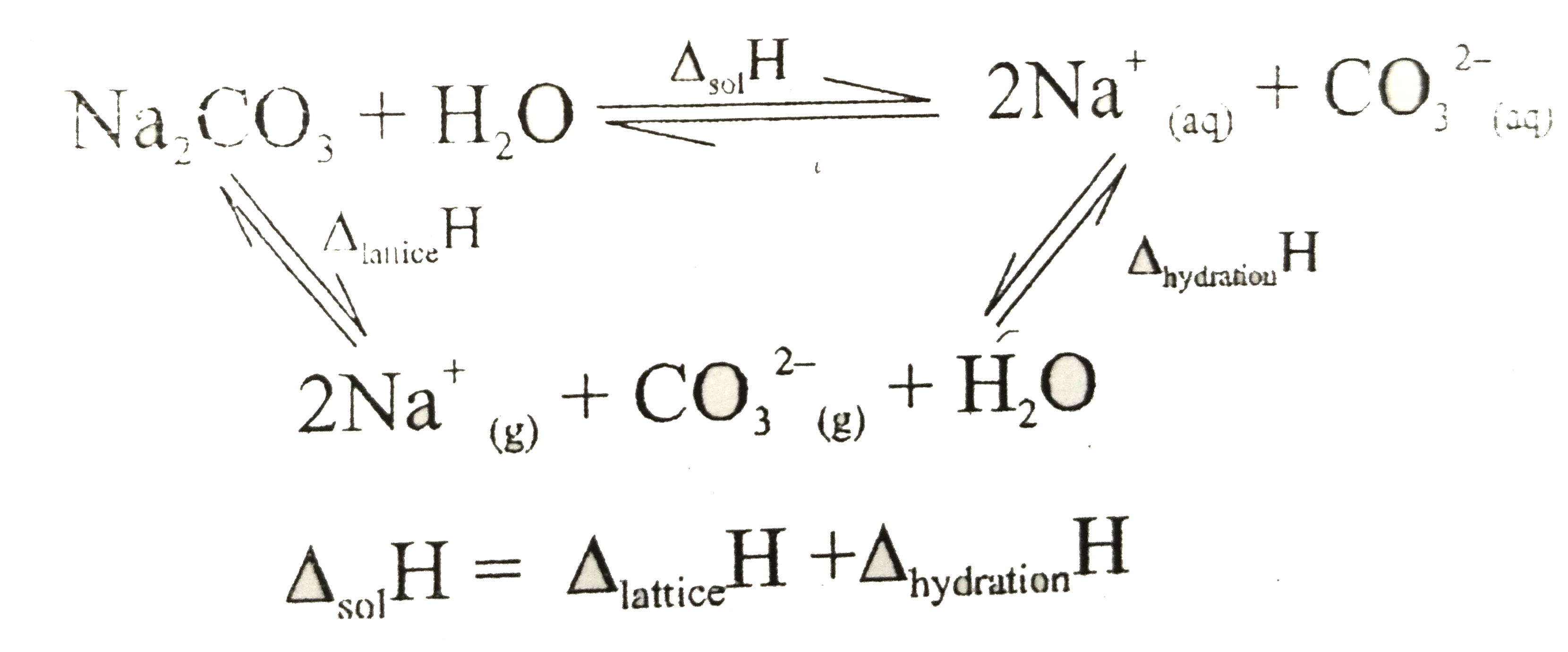 `=205+(-2 xx80-40)` `=+5` `IMPLIES `The process is endothermic So, the solubility will increase with increase in temperature . |
|