Saved Bookmarks
| 1. |
1.11g CaCl_(2) is added to water forming 500ml of solution 20ml of this solution is taken and diluted 10 folds Find moles of Cl ions in 2ml of diluted solution . |
|
Answer» Solution :`(1.11)/(111) = 0.01 "mol" CaCl_(2)` Moles of `CaCl_(2)` in 20mlsolution `= (0.01)/(500) xx 20 = (0.01)/(25)` In `200ml` solution moles of `CaCl_(2) = (0.01)/(25)` [Note Dilution does not change moles of solute] In `2ML` of DILUTE solution moles of `CaCl_(2)=(0.01)/(25/(2000))xx2=(0.01)/(2500)=8xx10^(-6)` . 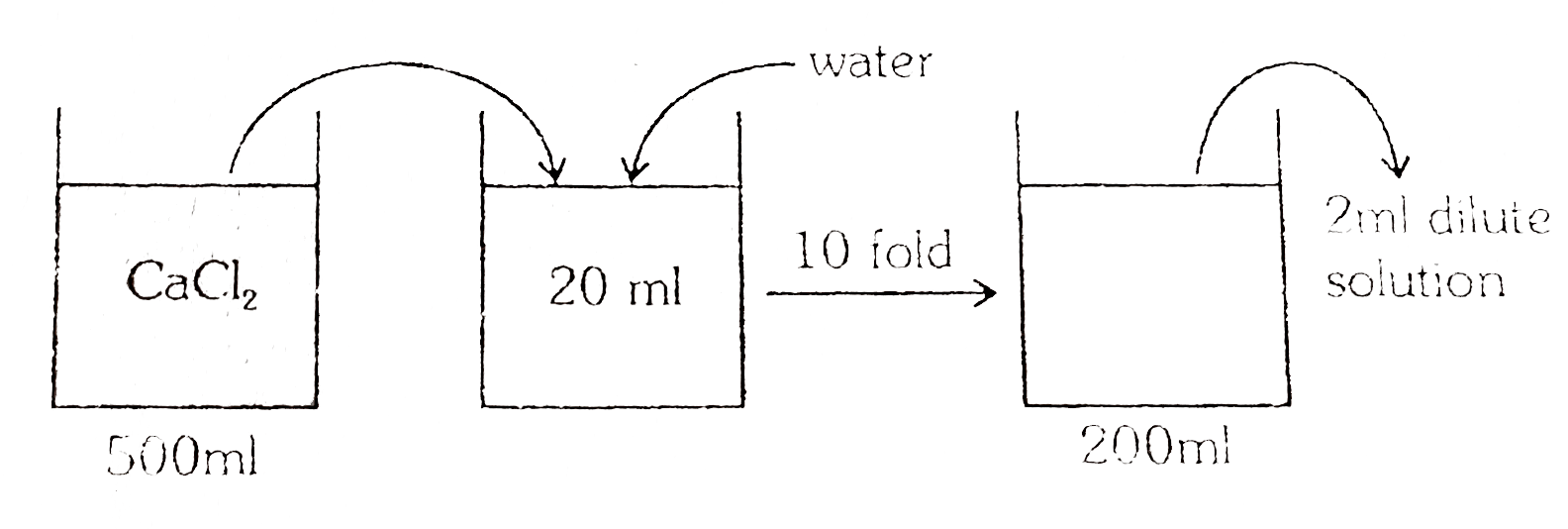
|
|
Discussion
No Comment Found
Related InterviewSolutions
- The weight of one molecule of compound C60H122 is
- Le
- Some important compounds of sodium, notes
- find the position of Zn30 in periodic table
- How to solve ion electron method
- Calculate the amount of water produced by the combustion of 16 g of methane
- Some MCQ between chapter 1and2
- Define reciprocal proportion
- What is the spectrum of hydrogen????
- I am not able to understand ch4 piz help me