Saved Bookmarks
| 1. |
1 mol of PCl_(5) , kept in a closed container of volume of 1 dm^(3) and was allowed to attain equilibrium at 423 K . Calculate the equilibrium composition of reaction mixture . (The K_(C) value for PCl_(5) dissociation at 423 K is 2 ) |
|
Answer» Solution :`PCl_(5) hArr PCl_(3) + Cl_(2)` Given that `[PCl_(5)]_("initial") =1` mol , `V = 1 dm^(3) , K_(C) = 2` 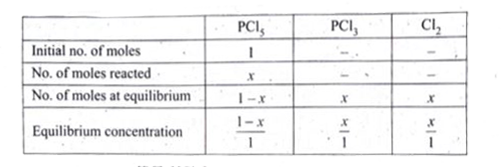 `K_(C) = ([PCl_(3)][Cl_(2)])/([PCl_(5)])` `2 = ( x xx x)/((1 - x))` `2 - 2x = x^(2)` `x^(2) + 2x - 2 = 0` Solution for a quadratic equation `ax^(2) + bx + c = 0` are `x = (- B pm sqrt(b^(2) - 4ac))/(2a)` `a = 1 . b = 2 . c = -2` `x = ( - 2 pm sqrt(4 - 4XX 1 xx (-2)))/(2 xx 1) = (-2 pm sqrt(12))/(2) = (-2 pm sqrt(4 xx 3))/(2)` `x = (-2 pm 2 sqrt3)/(2) = (- 2 + 2 sqrt3)/(2) , ( - 2 - 2 sqrt3)/(3)` `x = -1+ sqrt3 , - 1 - sqrt3` (SINCE x is +ve ) , `-1 - sqrt3` not possible `= - 1 + 1.732 = 0.732` `therefore` Equilibrium concentration of `[PCl_(5)]_(EQ) = (1- x)/(1) = 1 - 0.732 = 0. 268` M `[PCl_(3)]_(eq) = (x)/(1) = (0.732)/(1) = 0.732` `[Cl_(2)]_(eq) = (x)/(1) = (0.732)/(1) = 0.732` |
|
Discussion
No Comment Found
Related InterviewSolutions
- The weight of one molecule of compound C60H122 is
- Le
- Some important compounds of sodium, notes
- find the position of Zn30 in periodic table
- How to solve ion electron method
- Calculate the amount of water produced by the combustion of 16 g of methane
- Some MCQ between chapter 1and2
- Define reciprocal proportion
- What is the spectrum of hydrogen????
- I am not able to understand ch4 piz help me