Saved Bookmarks
| 1. |
1,2-Dimethylcyclohexene undergoes only trans-addition with HBr in non-polar solvents but both cis-and trans-additions occur with aq. Acid. Explain. |
|
Answer» Solution :This sterospecific reaction proceeds through a bridged cation like the bromonium ion where `H` replaces `Br`. The intermediate may actually be a `pi` -complex. In the absence of a solvent that can satbilise a FREE `R^(oplus), Br^(Ө)` attacks the protonated complex from the opposite side resulting in trans (anti) addition. In the case of a POLAR solvent such as `H_2 O`,the protonated complex collapses to the free `R^(oplus)`, which now REACTS from both sides (faces) and gives both `CIS-` and trans-additions. 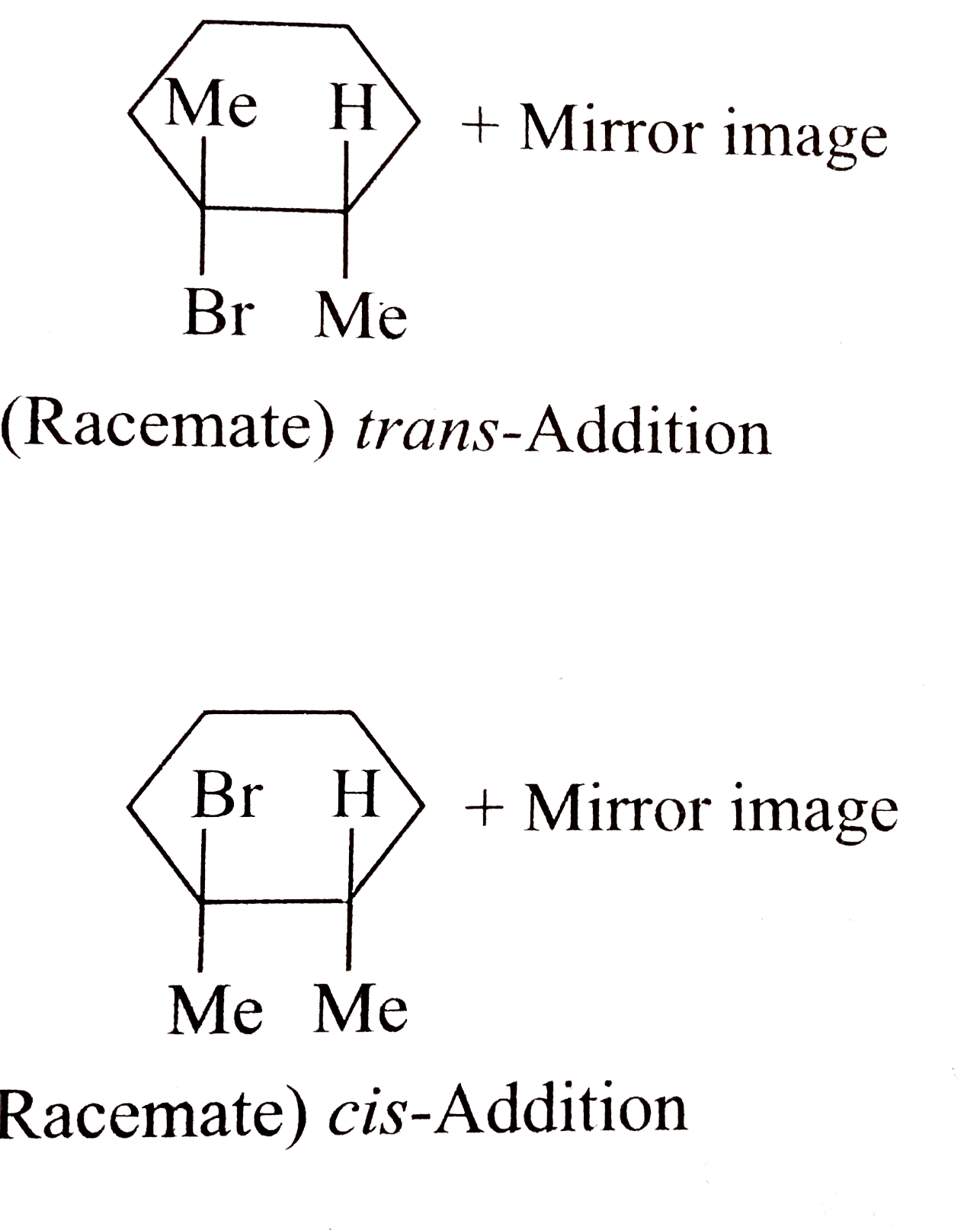 . .
|
|
Discussion
No Comment Found
Related InterviewSolutions
- The weight of one molecule of compound C60H122 is
- Le
- Some important compounds of sodium, notes
- find the position of Zn30 in periodic table
- How to solve ion electron method
- Calculate the amount of water produced by the combustion of 16 g of methane
- Some MCQ between chapter 1and2
- Define reciprocal proportion
- What is the spectrum of hydrogen????
- I am not able to understand ch4 piz help me