Saved Bookmarks
| 1. |
896 mL vapour of a hydrocarbon 'A' having carbon 87.80%and hydrogen 12.19 % weighs 3.29 g at STP, Hydrogenation of 'A' gives 2-methylpentane. Also 'A' on hydration in the presence of H_2SO_4 and HgSO_4 gives a ketone 'B' having molecular formula C_6H_12O. The ketone 'B' gives a positive iodoform test. Find the structures of 'A' and give the reactions involved. |
|
Answer» Solution :STEP 1 . To determine the molecular mass of hydrocarbon (A). 896 mL vapours of hydrocarbon (A) weigh at STP =3.28 g `therefore` 22700 mL vapours of A will weigh at STP `=(3.28xx22700)/896 "g mol"^(-1)=83.1 g` `therefore` Molecular mass of hydrocarbon (A) = `83.1 g mol^(-1)` Step 2. To determine the empirical formula of hydrocarbon (A). `{:("ELEMENT","% age","Atomic mass","RELATIVE ratio","Relative no. of atoms","Simplest ratio"),(C,87.8,12,7.31,1,3),(H,12.19,1,12.19,1.66,5):}` Thus, empirical formula of hydrocarbon (A) =`C_3H_5` and empirical formula mass = 12 x 3 + 5 x 1 = 41 u `therefore n="Molecular mass "/"Empirical formula mass "=83.1/41=2.02 approx 2` Thus, molecular formula of hydrocarbon (A)= 2 x Empirical formula = `2 xx C_3H_5 = C_6H_10` Step 3.To determine the structures of compounds (A) and (B) 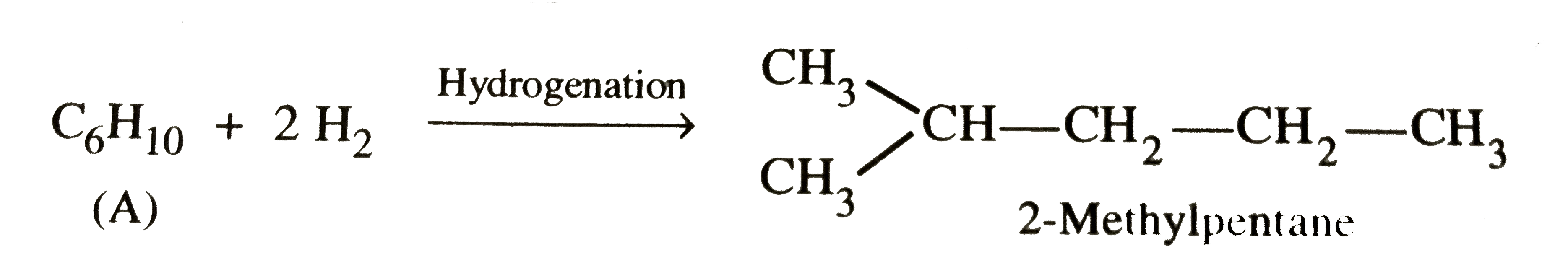 (i)Since hydrogenation of hydrocarbon (A) requires 2 MOLES of hydrogen to form 2-methylpentane, therefore, hydrocarbon (A) is an alkyne having five carbon atoms in a straigth chain and a methyl substitutent at POSITION 2. Thus, the two possible structures for the alkyne (A) are I and II :  (ii)Since addition of `H_2O` to alkyne (A) in presence of `Hg^(2+)` , gives a ketone which gives positive iodoform test, therefore, ketone (B) must be a methyl ketone , i.e., it must contain a `COCH_3` group. Now addition of `H_2O` to alkyne (II) should give a mixture of two ketone in which ketone (B) (Which shows +ve iodoform test ) predominates. 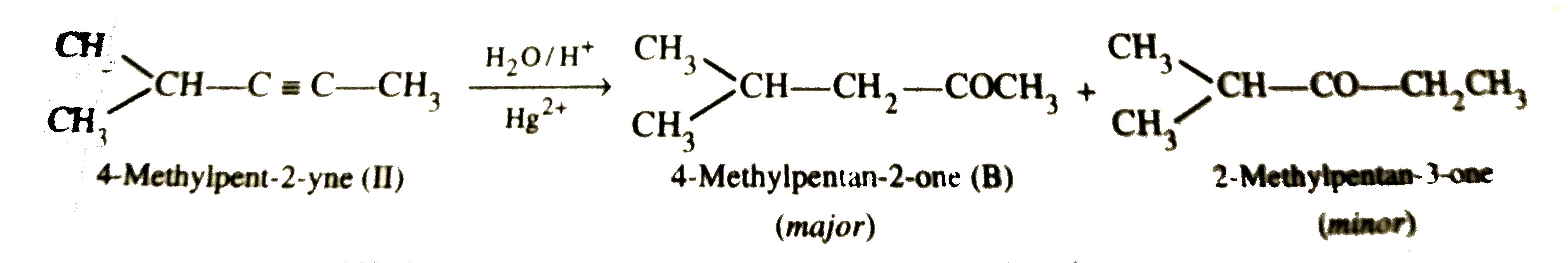 In contrast, addition of `H_2O` to alkyne (I) will give only one ketone , i.e., 4-methylpentan-2-one which gives iodoform test. 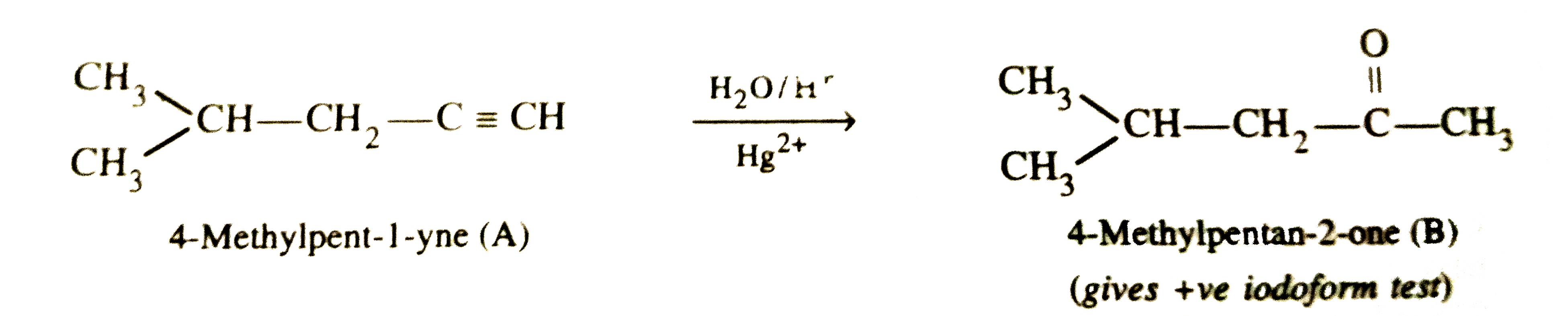 Thus, hydrocarbon (A) is 4-methylpent-1-yne. |
|
Discussion
No Comment Found
Related InterviewSolutions
- The weight of one molecule of compound C60H122 is
- Le
- Some important compounds of sodium, notes
- find the position of Zn30 in periodic table
- How to solve ion electron method
- Calculate the amount of water produced by the combustion of 16 g of methane
- Some MCQ between chapter 1and2
- Define reciprocal proportion
- What is the spectrum of hydrogen????
- I am not able to understand ch4 piz help me