Saved Bookmarks
| 1. |
(a) Account for the following : (i) Tendency to show - 2 oxidation state decreases from oxygen to tellurium. (ii) Acidic character increases from HF to HI. (iii) Moist SO_2 gas acts as a reducing agent. (b) Draw the structure of an oxoacid of phosphorus containing P - 0 - P linkage. (c) Complete the following equation : XeF_(2) +H_(2) O to OR (a) Among the hydrides of group 16, write the hydride (i) which is a strong reducing agent ? (ii) which has maximum bond angle ? (iii) which is most thermally stable ? Give suitable reason in each. (b) Complete the following equations : AgNO_(3) +H_(2) O + H_(3) PO_(2) to (c ) CI_(2) + underset("(Cold and dilute)")NaOH to |
|
Answer» Solution :(a) (i) As we move from top to bottom in group 16, the metallic character increases. Thus Te behaves as a METAL. It has no tendency to gain the electrons. Thus, its tendency to show - 2 OXIDATION state decreases. (ii) Bond length between Hand the HALOGEN increases as we move from F to I. Thus, hydrogen is less strongly bonded to halogen and is available for reduction. (iii) It gets oxidised to sulphuric acid and therefore acts as a reducing AGENT. `SO_(2) + H_(2) O+ O to H_(2) SO_(4) +O` (b) 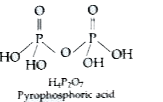 (c ) `2XeF_(2) + 2H_(2) O to 2Xe + 4 HF + O_(2) ` OR (a) (i) `H_2 Te` is the strongest reducing agent because the thermal stability is the MINIMUM. (ii) `H_2 O` has the maximum bond angle of 104.5°. As the size of group 16 element increases down the group, the electronegativity decreases. Group 16 element is not able to hold the lone pair of electrons to itself and lone pair-lone pair repulsions increase. Therefore `H_2 O` has the maximum angle and `H_2Te` the minimum angle. (iii) `H_(2) O` is thermally most stable because it has the maximum enthalpy of dissociation. (b) `Ag NO_(3) + H_(2) O+H_(3) PO_(2) to 4Ag + 4HNO_(3) +H_(3) PO_(4)` (c ) `CI_(2) + 2NaOH underset("dilute") overset("Cold and") (to) Na CI + Na CIO + H_(2) O` |
|
Discussion
No Comment Found
Related InterviewSolutions
- Which of the following compounds is not cleaved by HI even at 525 K ?
- To a 25 mL H_(2)O_(2) solution excess of an acidified solution of potassium iodide was added. The iodine liberated required 20 " mL of " 0.3 N sodium thiosulphate solution Calculate the volume strength of H_(2)O_(2) solution.
- The suggested mechanism of a reaction is : (a) A+BhArrD("fast) "(b)A+Drarr2C("slow")Write the balanced equation of the reaction if its experimentally deduced rate equation is , rate k=[A]^(2)[B] Find the intermediate formed during the course of the reaction . Does the predicted rate law from the mechanism match the experimental rate law ?
- Which of these changes with time for a first-order reaction A Rate of reaction B . Rate constant C . Half-life
- What is the hybridisation of central atom in the product obtained along with hydrofluoric acid when complete hydrolysis of Xenon Hexa Fluoride takes place ?
- Which of the following amino acid forms sulphide bond in polypeptide
- Which of following pair is Diastereomers:
- What is the major product of the following reaction CH_3C-=C-CH_2-CH_3overset("1 mole of " Cl_2)to
- Which polymer is used in petrol tank linings ?
- Which of the following carbohydrates are branched polymer of glucose ?