Saved Bookmarks
| 1. |
a. Acid halides, anhydrides, esters and amides, all of them contain (C=O) group but none of them gives test for (C=O) group, i.e., they do not form oximes, hydrazones, and DNP derivatives, etc. Why? b. Arrange the following in the decreasing order of nucleophilic addition. i. (1) -CHO (2) -COCH_(3) (3) -COOH (4) -COCI (5) -CONH_(2) (6)-COOCH_(3) (7)-COO^(Θ) ii. (1) CH_(3)CHO (2) CH_(3)COCH_(3) (3) HCHO (4)CH_(2)H_(5)CH_(2)COCH_(3) iii. (1) CH_(3)COCH_(3) (2)C_(6)H_(5)COCH_(3) (3) C_(6)H_(5)COC_(6)H_(5) (4) C_(6)H_(5)CH_(2)COCH_(3) iv. (1)C_(6)H_(5)CHO (2) rho-CH_(3).C_(6)H_(4).CHO (3)rho-OH.C_(6)H_(4).CHO (4) rho-NO_(2).C_(6)H_(4).CHO (5) rho-CI.C_(6)H_(4).CHO v. (1) HCHO (2) CH_(3)CHO (3) CH_(3)COCH_(3) (4) CI_(3)C CHO c. Arrange the following in the increasing order of ease of hydrolysis. (1)C CH_(3)COOC_(2)H_(5) (2) CH_(3)COCI (3) (CH_(3)CO)_(2)O (4) CH_(3)CONH_(2) d. Arrange the following in the increasing order of ease of acid-catalysed esterification of: i. ii. (1) CH_(3)CH_(2)COOH (2) (CH_(3))_(2)CHCOOH (3) (CH_(3))_(3)C COOH iii. (1) CH_(3)CH_(2)CH_(2)OH (2) CH_(3)CHOHC_(2)H_(5) (3) (CH_(3))_(2)C(OH)C_(2)H_(5) e. Arrange the following esters in decresing order of ease of alkaline hydrolysis. i. (1) HCOOCH_(3) (2) CH_(3)COOCH_(3) (3) (CH_(3))_(3)C COOCH_(3) (4) (CH_(3))_(2)CHCOOCH_(3) ii. (1) CH_(3)COOCH_(3) (2) CH_(3)COOC(CH_(3))_(3) (3) CH_(3)COOCH(CH_(3))_(2) (4) CH_(3)COOCH_(2)CH_(3) iii. (1) C_(6)H_(5)COOCH_(3) (2) rho-CH_(3)O-C_(6)H_(4)-C COOCH_(3) (3) rho-NO_(2)-C_(6)H_(4)-COOCH_(3) (4) rho-Cl-C_(6)H_(4)-COOCH_(3) |
|
Answer» Solution :All the compounds do not contain a true `(C=O)` bond as shown by their resonance structures and hence do not give any test of `(C=O)` group. However, all the ACID derivatives react differently (nucleophilic acyl substitution) with ammonia derivatives e.g., I. REACTION of acid halide with ammonia derivative: i. 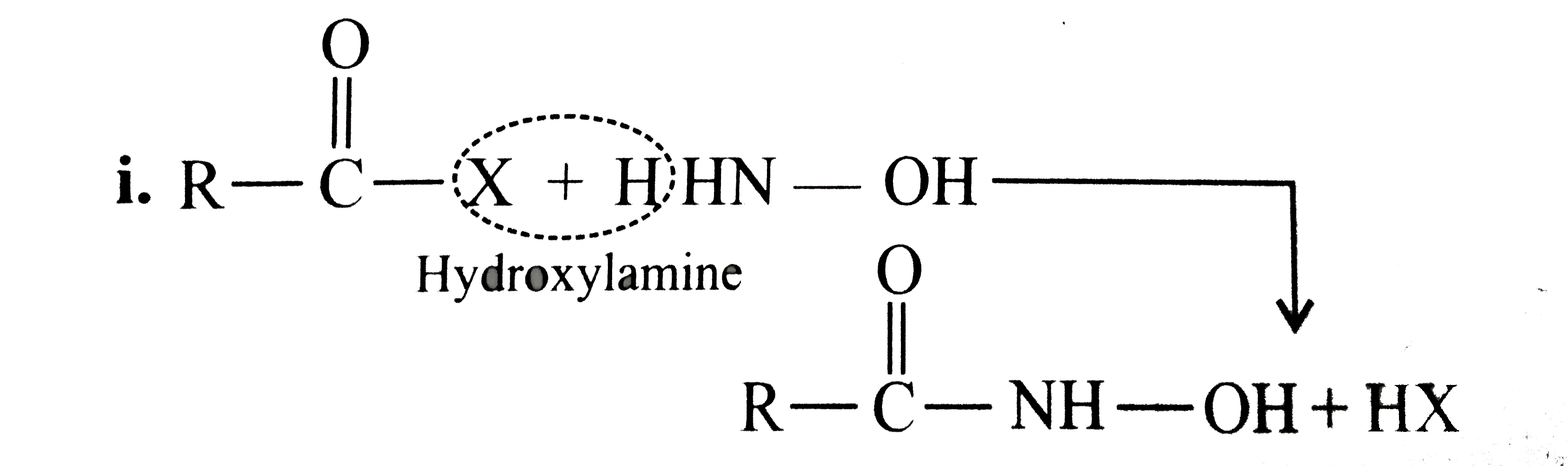 ii. 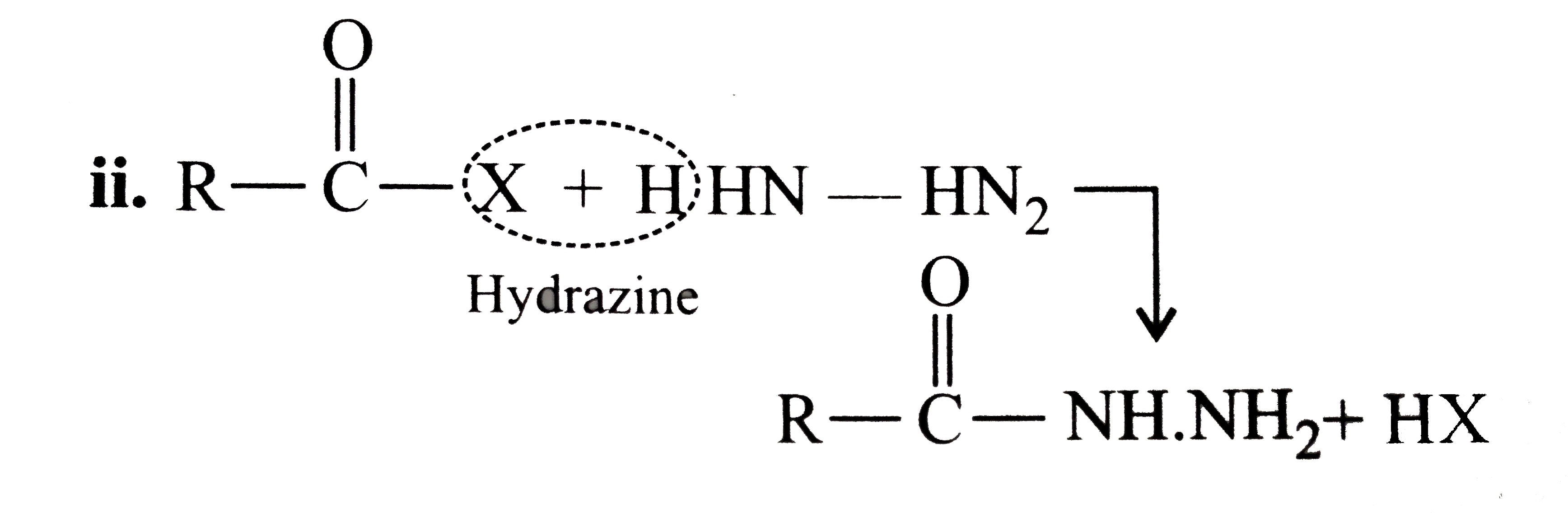 iii. 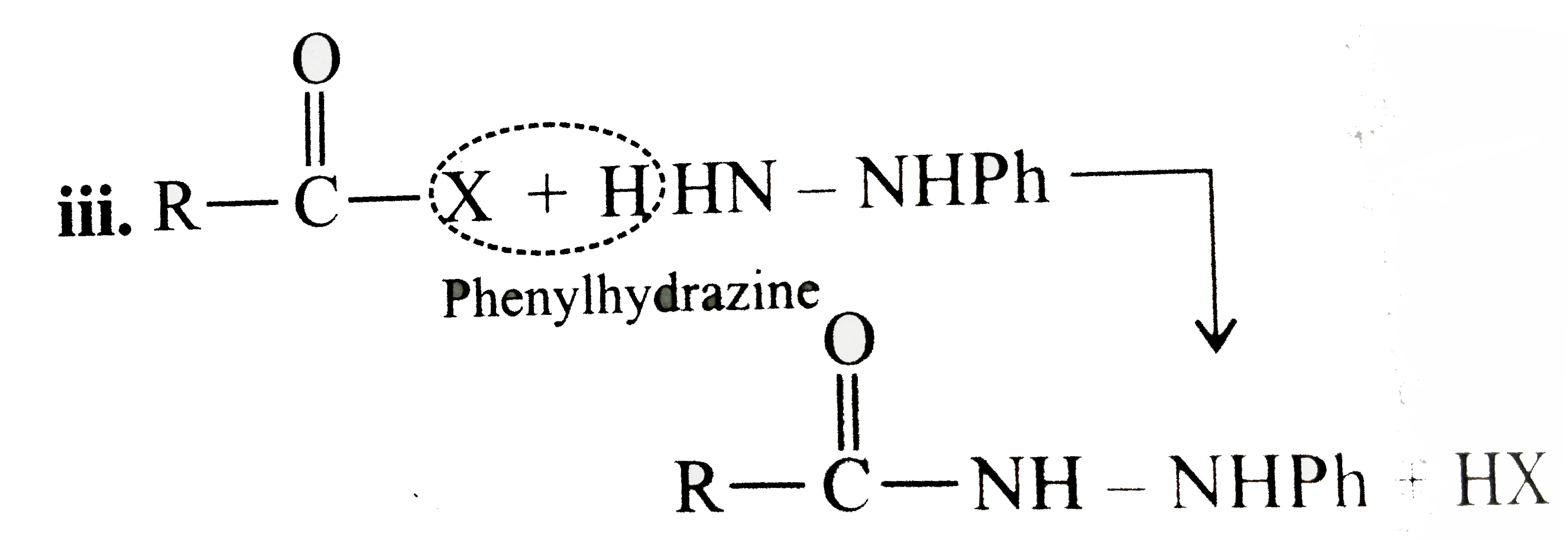 II. Reaction of anhydride with ammonia derivative: i. 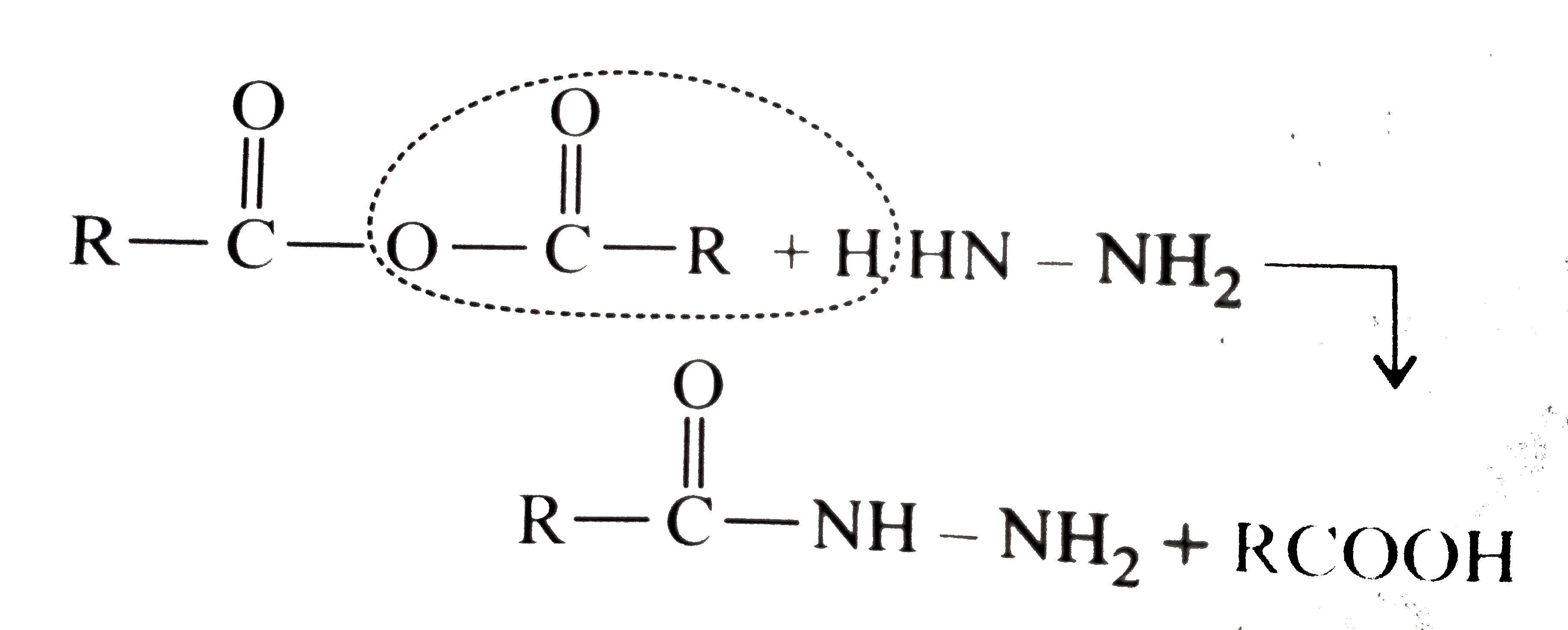 ii. 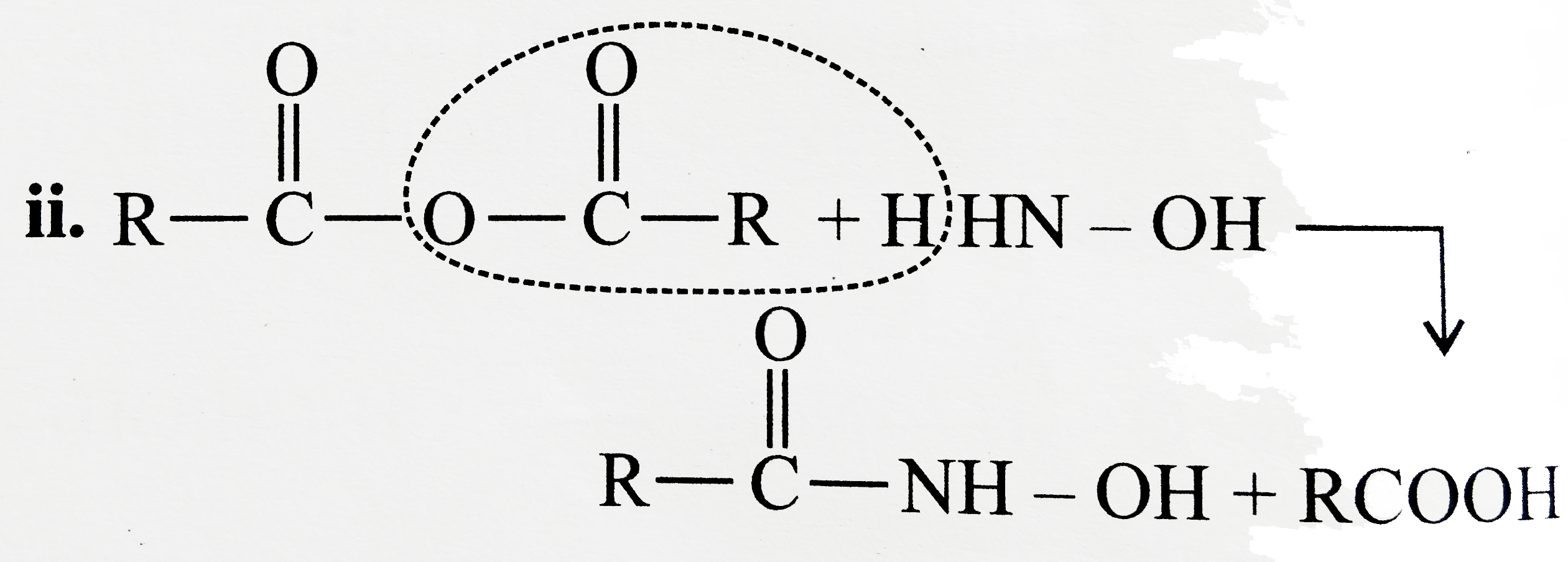 III. Reaction of esters with ammonia derivative: i.  ii. 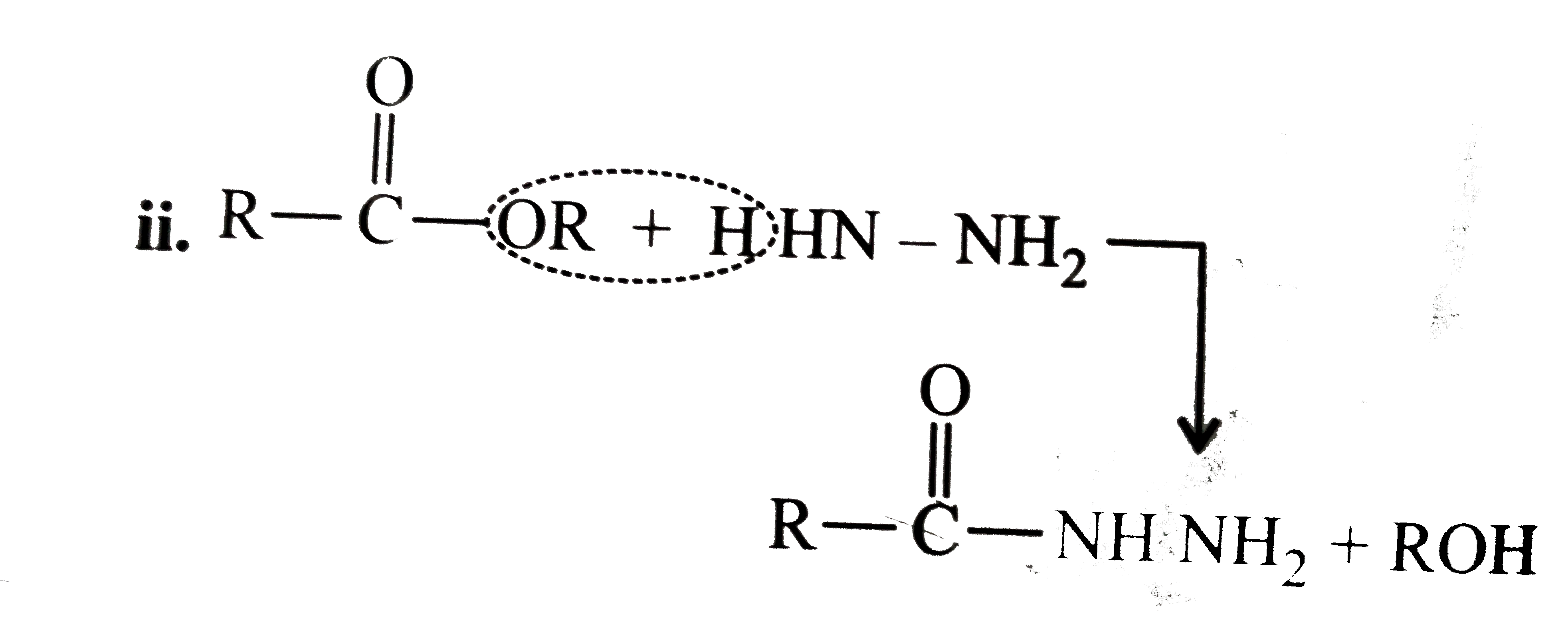 IV. Reaction of amide with ammonia derivative: i. 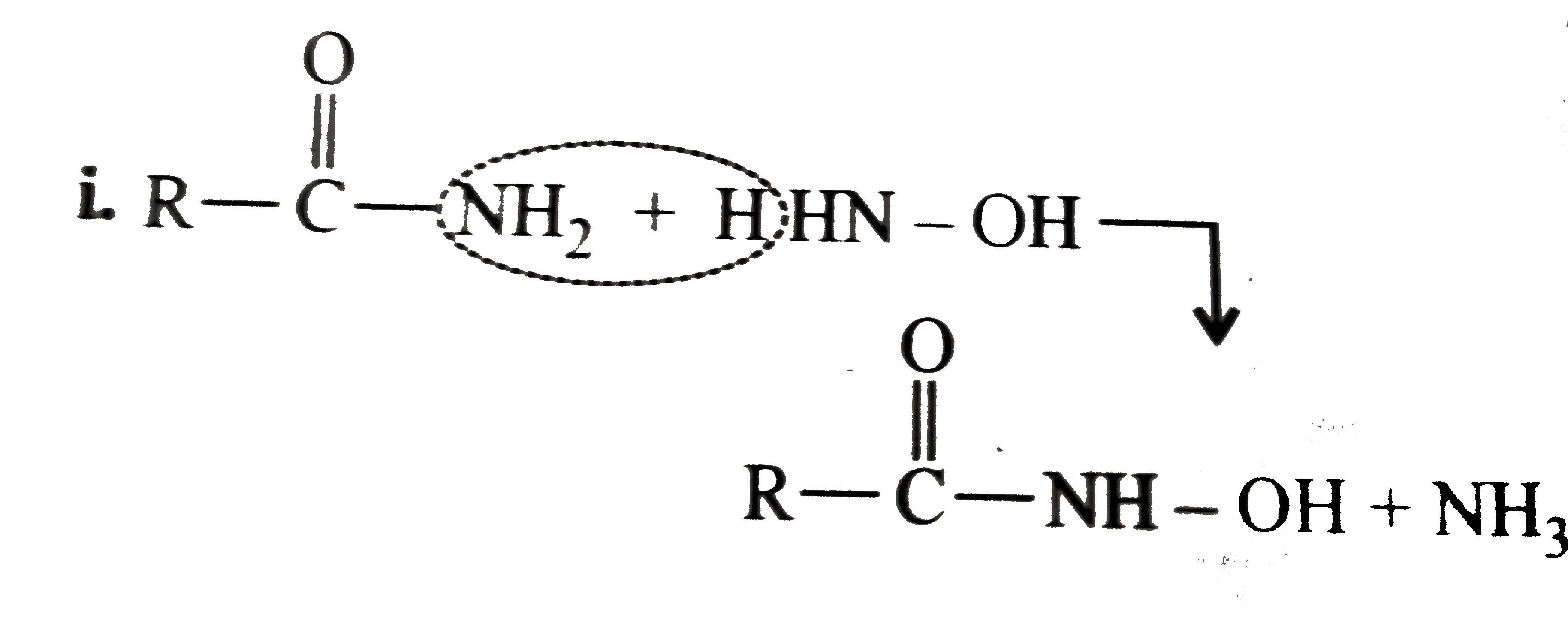 ii. 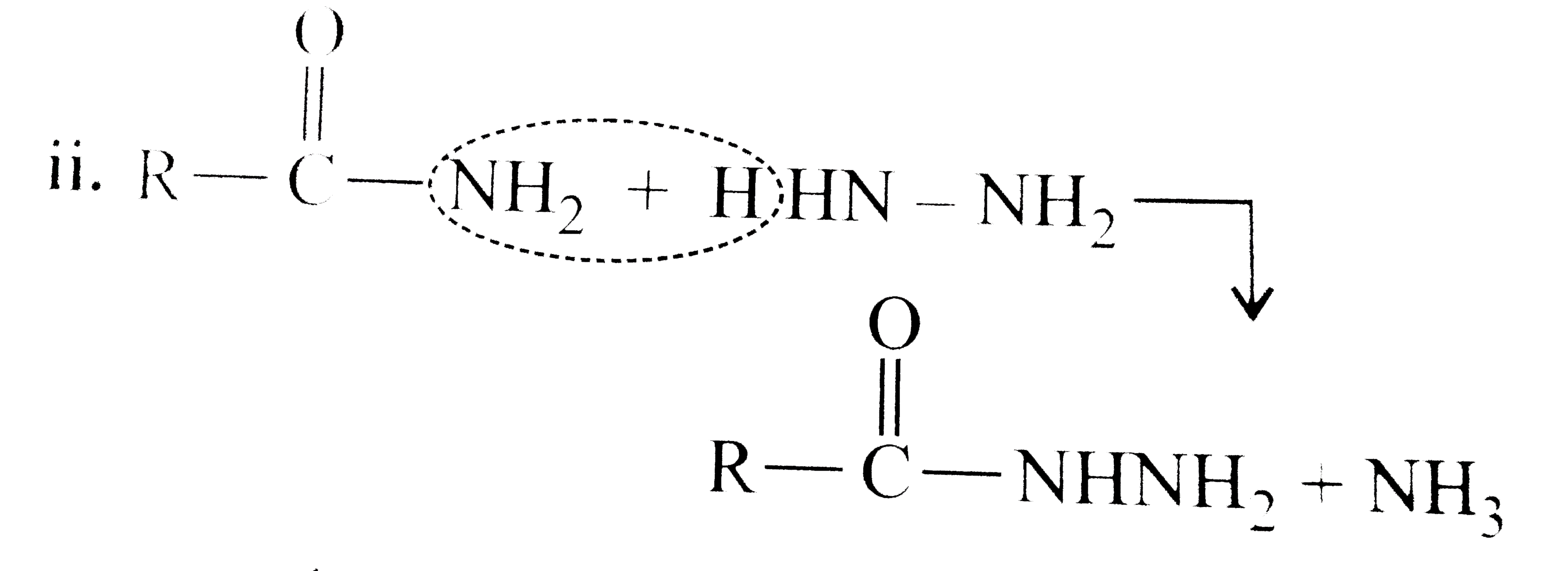 b. i. `1gt2gt4gt6gt5gt3gt7` (Aldehyde gt Ketone gt Acid chloride gt Ester gt Amidegt Acid gt Acid ion) ii. `3gt1gt2gt4` iii. `4gt1gt2gt3` More the `bar(e)-`withdrawing group, faster in the NA. In (4), Ph group is `bar(e)-`withdrawing by `-I` effect (+R cannot OCCUR, SINCE there is no extended conjugation)  iv. `4gt5gt1gt2gt3` `[rho-N NO_(2)-(-I and -R), rho-C1 (-I) gt "Standard" gt rho-Me (+I and H.C.) gt rho-OH (+R and -I)]` v. `4gt5gt1gt2gt3 [(C1_(3)C-CHO) (-I "of" 3C1)gtHCHOgtCH_(3)CHOgtCH_(3)COCH_(3)]` c. `2gt3gt1gt4` (Acid chloride gt Anhydride gt Ester gt Amide) d. As the size of substituents on the `alpha-`C increases, the tetrahedrally bonded intermediate becomes more crowded. Greater the crowding, slower is the reaction. i. `1gt2gt3` ii. `1gt2gt3` iii. `1gt2gt3` e. i. `1gt2gt4gt3` ii. `1gt4gt3gt2` iii. `3gt4gt1gt2` |
|
Discussion
No Comment Found
Related InterviewSolutions
- Which of the following compounds is not cleaved by HI even at 525 K ?
- To a 25 mL H_(2)O_(2) solution excess of an acidified solution of potassium iodide was added. The iodine liberated required 20 " mL of " 0.3 N sodium thiosulphate solution Calculate the volume strength of H_(2)O_(2) solution.
- The suggested mechanism of a reaction is : (a) A+BhArrD("fast) "(b)A+Drarr2C("slow")Write the balanced equation of the reaction if its experimentally deduced rate equation is , rate k=[A]^(2)[B] Find the intermediate formed during the course of the reaction . Does the predicted rate law from the mechanism match the experimental rate law ?
- Which of these changes with time for a first-order reaction A Rate of reaction B . Rate constant C . Half-life
- What is the hybridisation of central atom in the product obtained along with hydrofluoric acid when complete hydrolysis of Xenon Hexa Fluoride takes place ?
- Which of the following amino acid forms sulphide bond in polypeptide
- Which of following pair is Diastereomers:
- What is the major product of the following reaction CH_3C-=C-CH_2-CH_3overset("1 mole of " Cl_2)to
- Which polymer is used in petrol tank linings ?
- Which of the following carbohydrates are branched polymer of glucose ?