Saved Bookmarks
| 1. |
A compound formed by elements X and Y has a cubic structure in whch X atoms are at the corner of the cube and also at the face centers. Y atoms are present at the body center and at the edge center of the cube. If all the atoms are removed from one of the axis passing through one of the face centers of the cube, then formula of the compound is |
|
Answer» XY 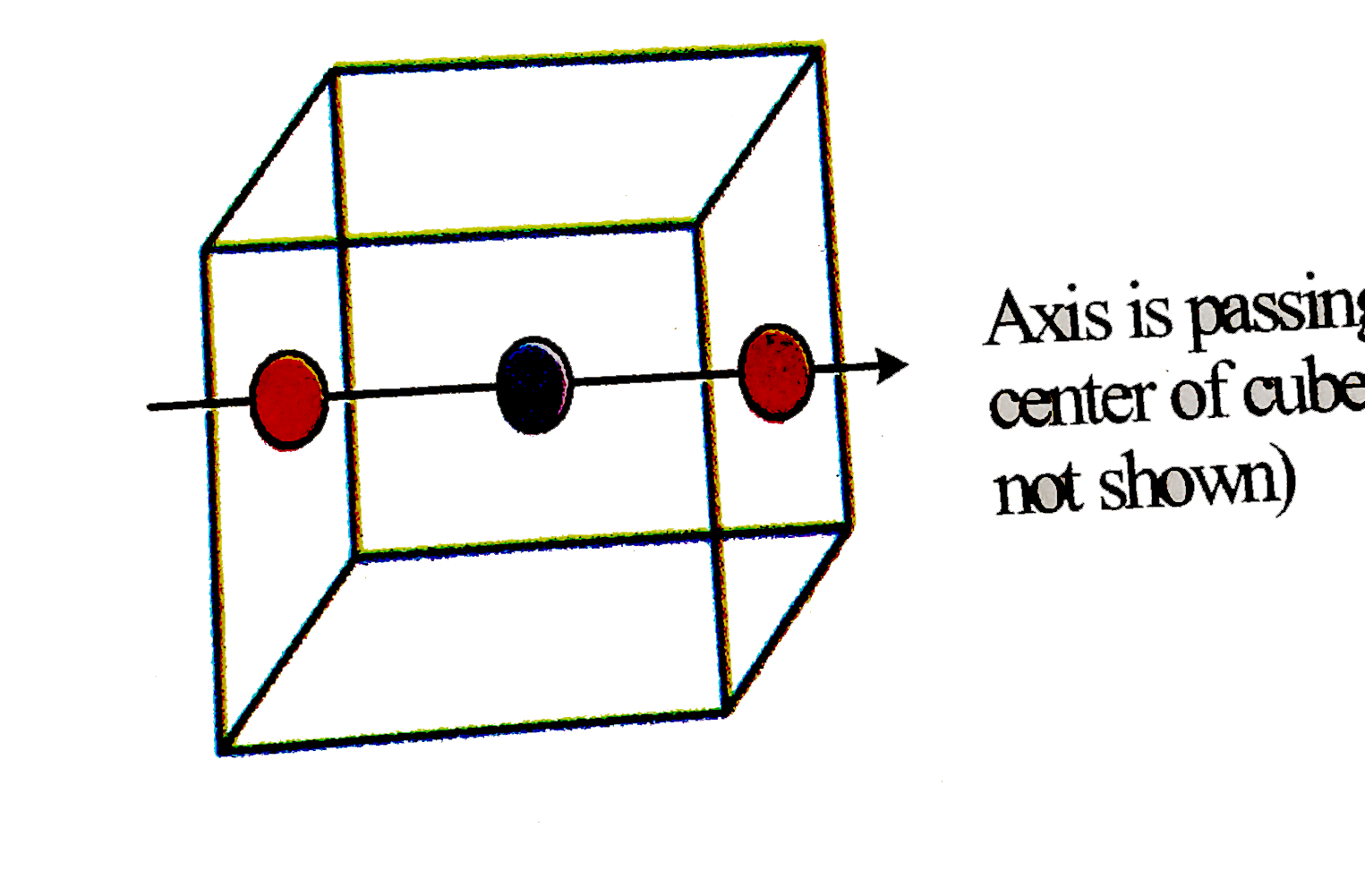 `YrArr1` body CENTER atom is removed `XrArr2` FACE center atoms are removed Formula : `Z_(eff(X))=3,Z_(eff(Y))=3` Formula is `X_(3)Y_(3)` or 3XY. |
|
Discussion
No Comment Found
Related InterviewSolutions
- The weight of one molecule of compound C60H122 is
- Le
- Some important compounds of sodium, notes
- find the position of Zn30 in periodic table
- How to solve ion electron method
- Calculate the amount of water produced by the combustion of 16 g of methane
- Some MCQ between chapter 1and2
- Define reciprocal proportion
- What is the spectrum of hydrogen????
- I am not able to understand ch4 piz help me