Saved Bookmarks
| 1. |
A cyclic process 1rarr2rarr3rarr1 shown in P-T diagram is performed with a constant mass of an ideal gas. Show it on a (a) P-V diagram (b) V-T |
|
Answer» <P> Solution :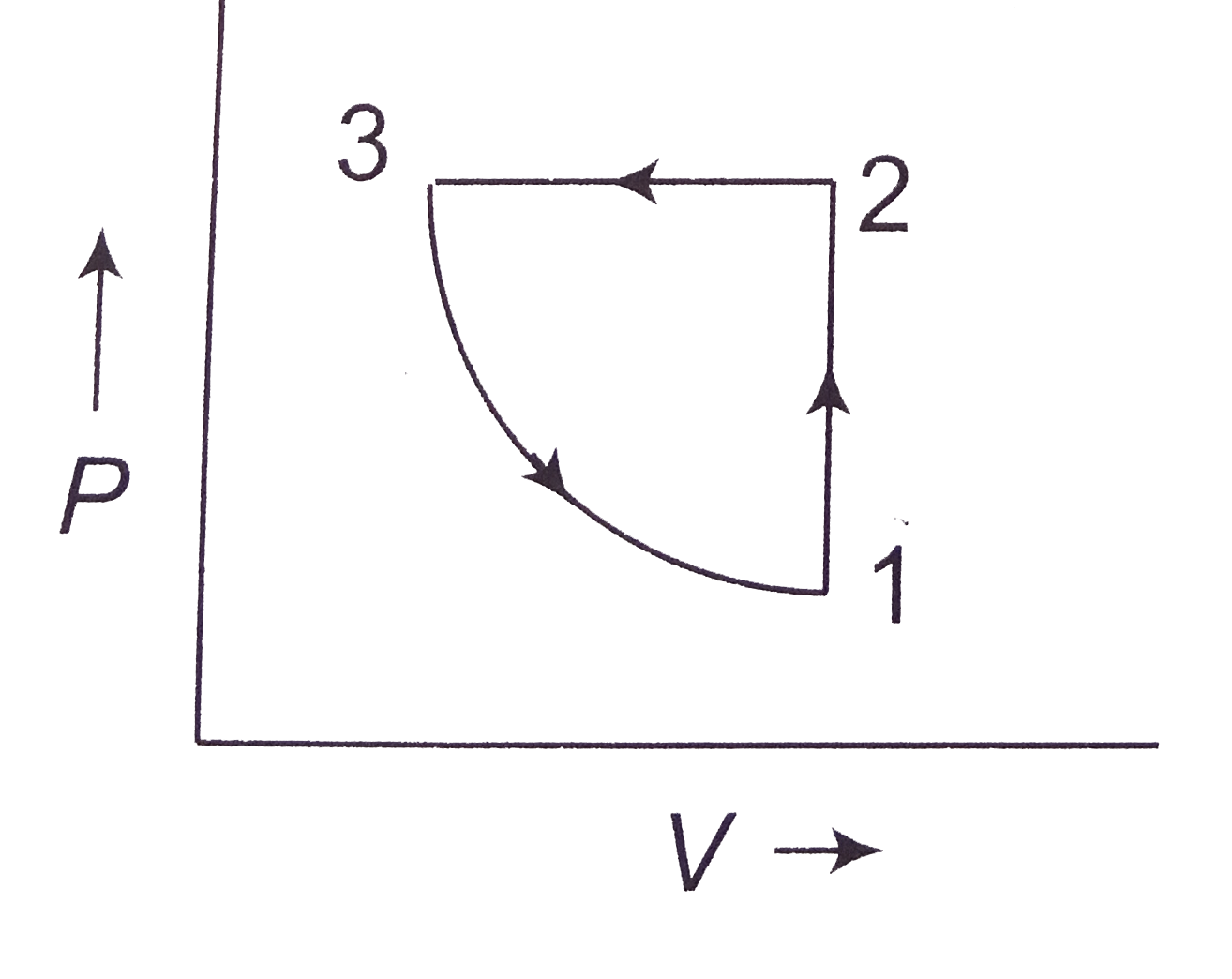 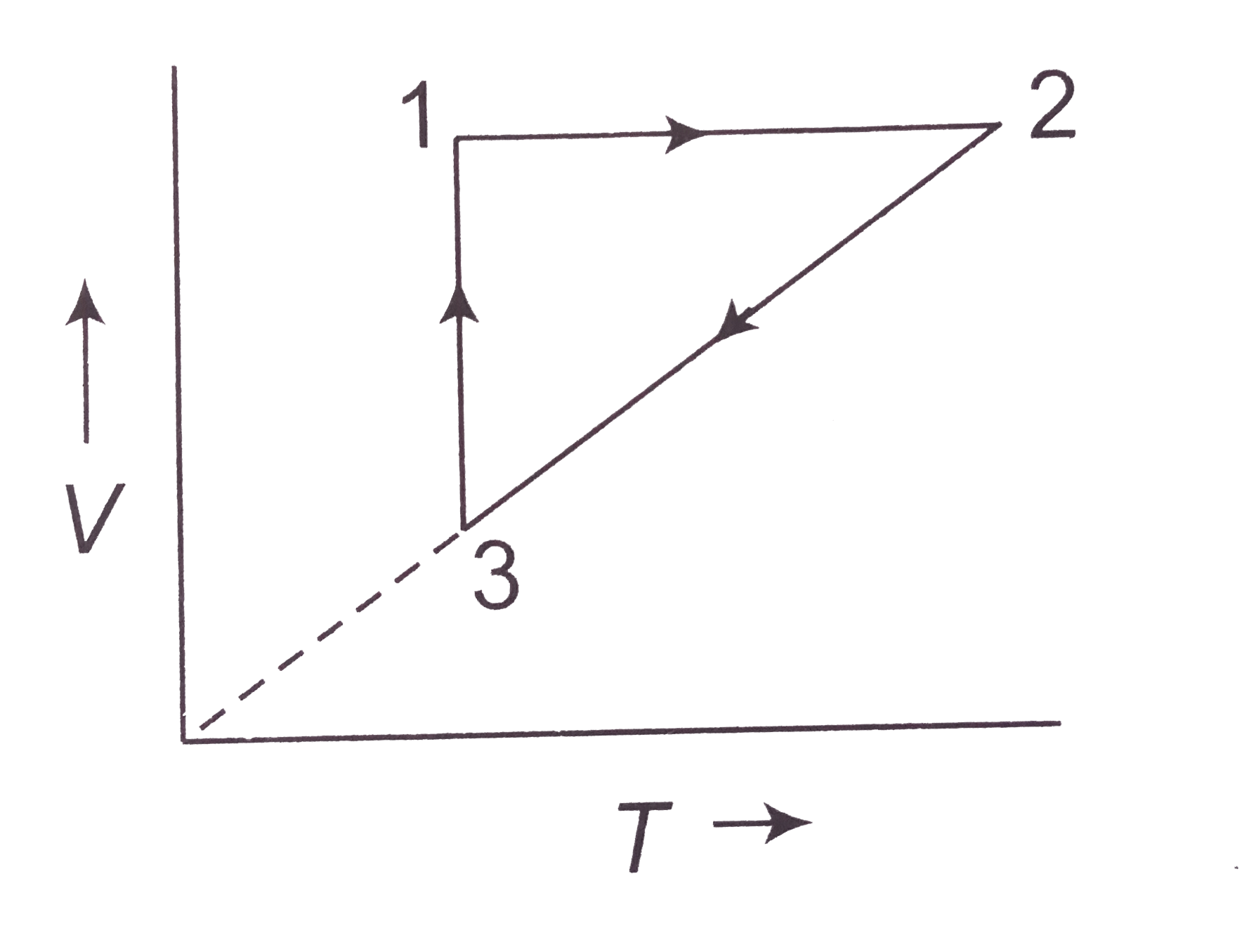 (a) `1rarr2` is an inclined line passing through origin i.e., it represents isochoric process (V: constant) `1rarr2` P is increasing `2rarr3`: Pressure is constant `PV=nRTimpliesVpropT` As TEMPERATURE is decreasing, HENCE, volume is decreasing. `3rarr1` Temperature is constant `P-V` diagram will be rectangular hyperbola. In `3rarr1`, pressure is decreasing The `P-V` diagram will be (B) `1rarr2`: Volume is constant, temperature is increasing `2rarr3`: P is constant , on `V-T` diagram `2rarr3` will straight line through origin. T is decreasing. `3rarr1`: T is constant, P is decreasint i.e., volume is increasig (since `PV=nRT`) |
|
Discussion
No Comment Found
Related InterviewSolutions
- What is uniform velocity
- Derive the speed time equation by calculas method
- 9×2y - 24x^2 +16y^3.factorise?
- Give me tips for preparation of physics??
- Advantage of friction
- Derivation of equation of Newton\'s Second Law of Motion that is F=ma
- Equation of projectile?
- Unit of Permeability
- Difference between vectr and scalar
- what is centripetal force ?