Saved Bookmarks
| 1. |
A metallic element crystallizes into a lattice containing sequenceof layers of ABABAB….. Any packing of spheres leaves out voids in the lattice. What precentage by volume of this lattice is empty space. |
|
Answer» Solution : ABAB….. Type of packing means hexagonal close packing. A UNIT cell ( with lifted layers)is shown in the FIG 1.61. suppose the radius of each sphere =r Volumeof the unit cell = Base area ` xx` height (h) Base area of regular hexagon = Area of SIX equilateral triangle s. each with side a ( i.e,2r) =` 6 xx sqrt3/4 a^(2)` Height h =2 ` xx` Distance between closest packed layers. ` 2xx SQRT(2/3) a` voluvme of unit cell =` ( 6 xx sqrt3/4 a^(2)) ( 2xx sqrt(2/3) a)= 3sqrt2 a^(3)` putting a = 2r, volume of the unit cell` =3 sqrt 2 ( 2r)^(3)= 24 sqrt(2) r^(3)` No. of atoms in hcp per unit cell =` 12xx 1/6` (corners)` + 2 xx 1/2` ( FACE centres)+ 3(in the body) = 6 Volume of six spheres ` = 6 xx 4/3 pi r^(3) = 8 pi r ^(3)` packing fraction = `( 8 pi r^(3))/(24 sqrt2 r^(3)) = pi/( 3sqrt2)= 3.143/(3xx 1.414)= 0.74` % volume occupied = 74 % % volume empty space = 26 % 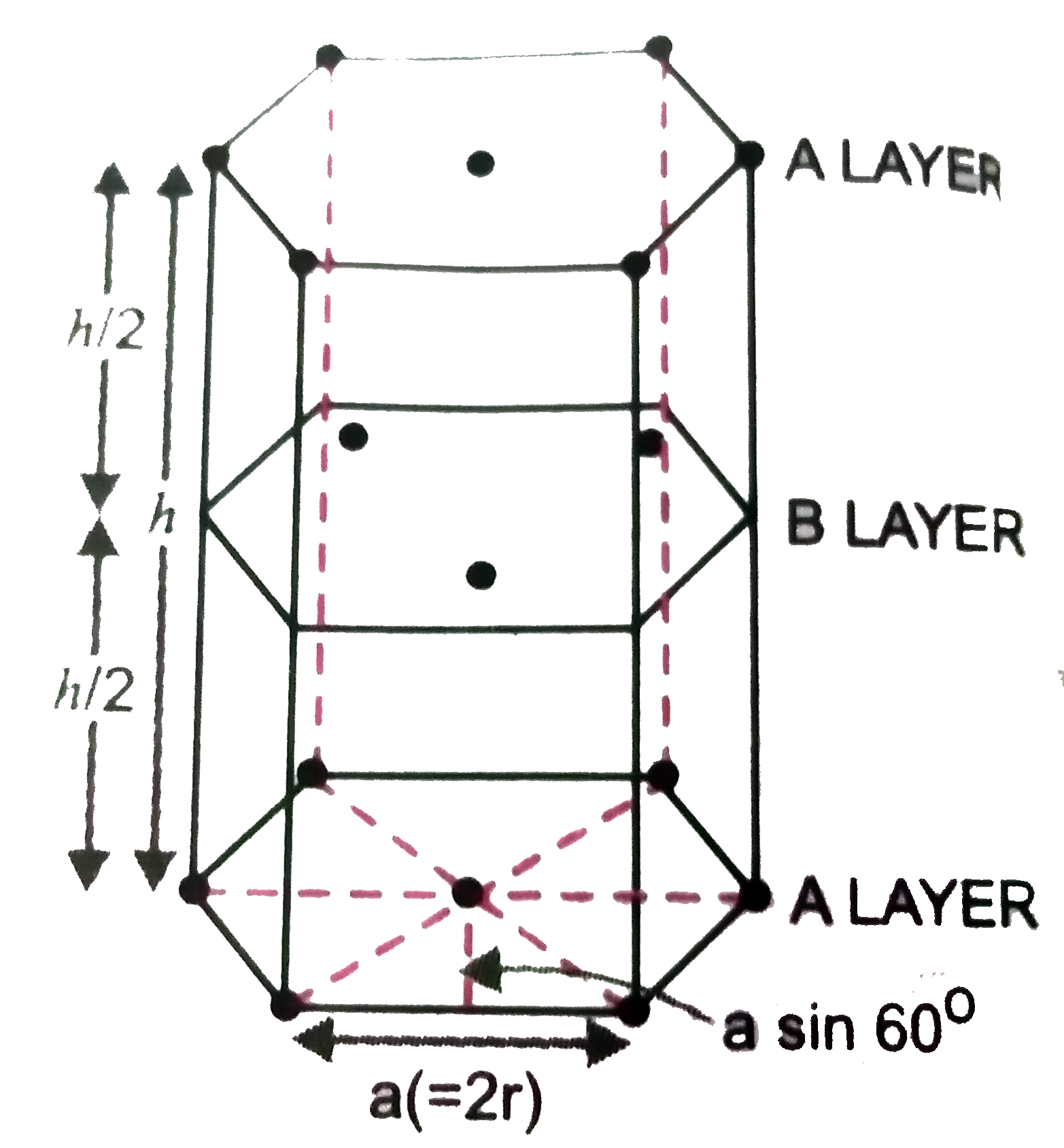
|
|
Discussion
No Comment Found
Related InterviewSolutions
- The weight of one molecule of compound C60H122 is
- Le
- Some important compounds of sodium, notes
- find the position of Zn30 in periodic table
- How to solve ion electron method
- Calculate the amount of water produced by the combustion of 16 g of methane
- Some MCQ between chapter 1and2
- Define reciprocal proportion
- What is the spectrum of hydrogen????
- I am not able to understand ch4 piz help me