Saved Bookmarks
| 1. |
Alkene(A) and (B) yield the same alcohol (C) on hydration. On vigorous oxidation with KMnO_4, (A) gives a carbonyl compound (D) and an acid (E) each containing four carbon atoms. On the other hand (B) gives an aicd (F) and a carbonyl compound (G), In (G), no two identical groups are attached to the same carbon atom. Give structures of (A) to (G) with proper resoning. |
|
Answer» Solution :Akene (A) on OXIDATION gives a CARBONYL compound (D) and an acid (E). Each having for carbon atoms. THUS, the probable structure of (A) may be, `CH_3-CH_2underset(CH_3)underset(|)C=CH-CH_2-CH_3` or `CH_3-CH_2-underset(CH_3)underset(|)C=CH-underset(CH_3)underset(|)CH-CH_3`. Alkene (B) gives an acid (F) and a carbonyl compound (G). Alkene (B) gives same alcohol on hydration and is ISOMERIC to (A) the carbonyl compound (G) has no carbon atom having two identical groups. Thus, the structure of (B) should be , `underset((B))(CH_3-CH=underset(CH_3)underset(|)C-CH_2-CH_2-CH_2-CH_3)` The alkene (A) should also posses the corresponding structure, thus , structures of (A) is ,`underset((A))(CH_3-CH_2-underset(CH_3)underset(|)C=CH-CH_2-CH_2-CH_3)` REACTIONS : 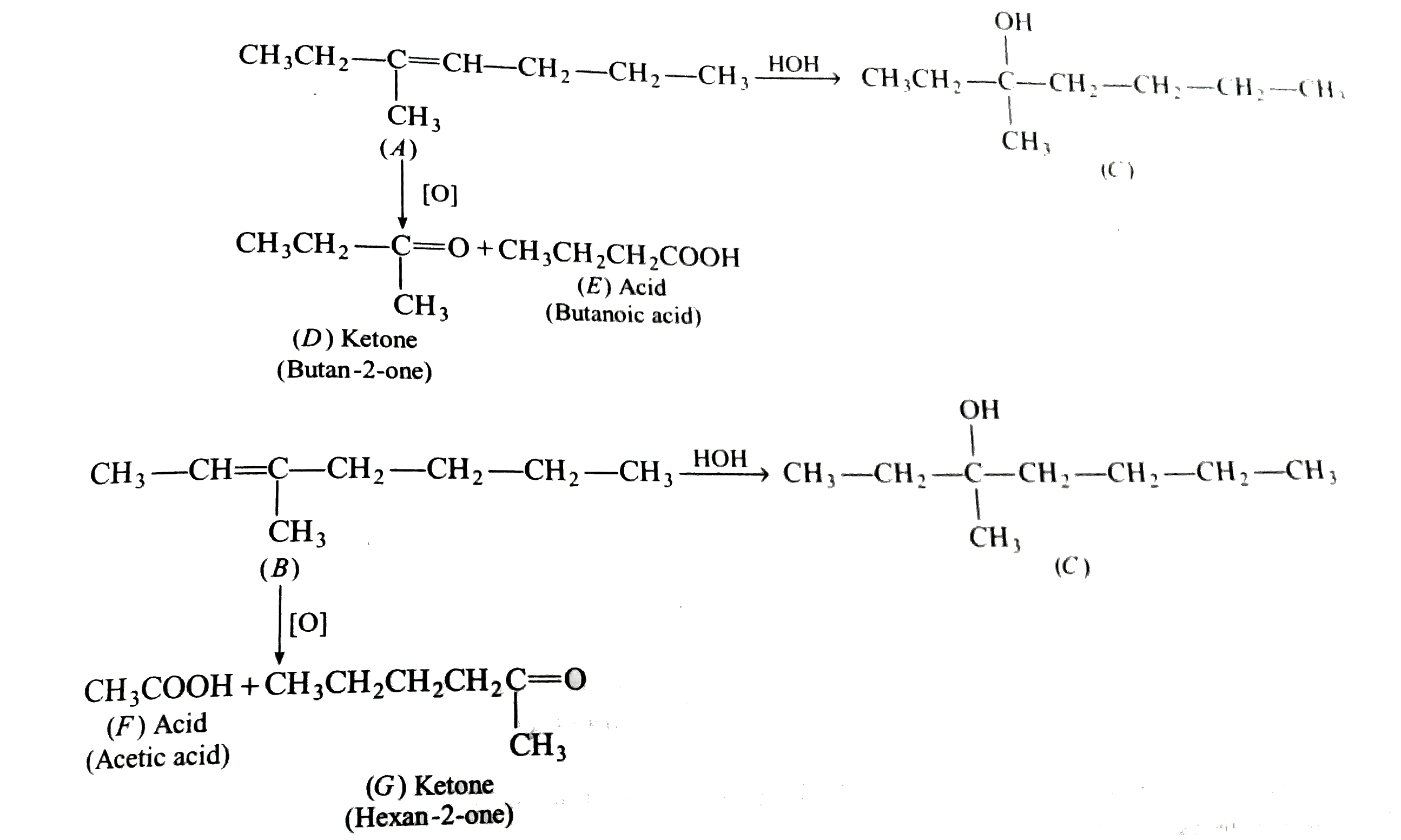
|
|
Discussion
No Comment Found
Related InterviewSolutions
- The weight of one molecule of compound C60H122 is
- Le
- Some important compounds of sodium, notes
- find the position of Zn30 in periodic table
- How to solve ion electron method
- Calculate the amount of water produced by the combustion of 16 g of methane
- Some MCQ between chapter 1and2
- Define reciprocal proportion
- What is the spectrum of hydrogen????
- I am not able to understand ch4 piz help me