Saved Bookmarks
| 1. |
Question : What isdenoted byand Y in the given graph ? |
|
Answer» `{:(""X,""Y),("Activation energy without enzyme", "Activation energy with enzyme"):}` 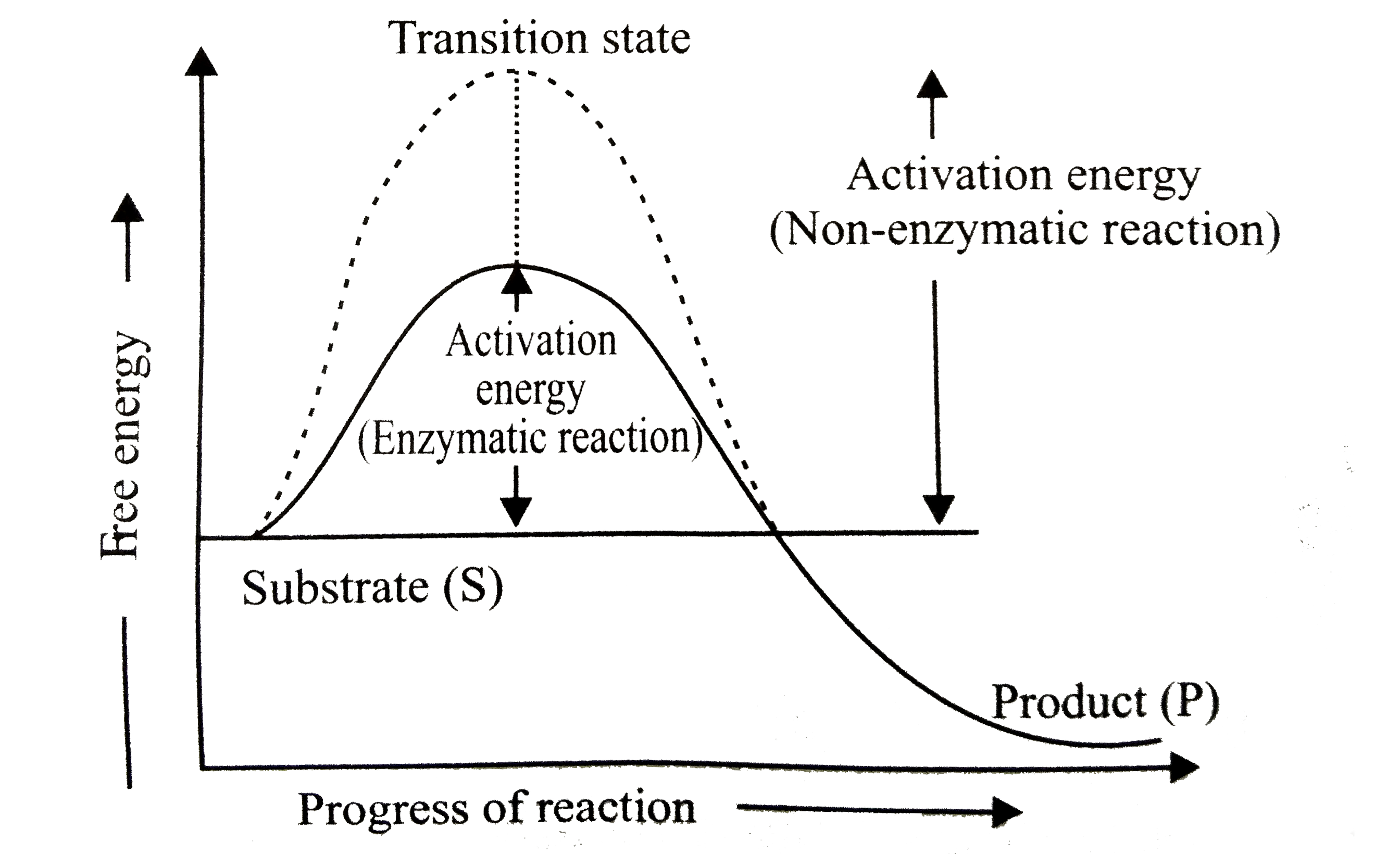
|
|
Discussion
No Comment Found
Related InterviewSolutions
- What is epidermal tissue system?
- Minimal coiling of chromosome
- Ncert question ch 16
- Ncert question of biology class 11
- What is phyllotaxy??????
- What is mycorrhizal one mark
- What is alternation of generation
- How is deuteromycetes different from other fungi?
- How can u classify leaves?
- Define nucleoplasm