Saved Bookmarks
| 1. |
What are the steps involved in the extraction of aluminium from bauxite? |
|
Answer» Solution :Aluminium is usually EXTRACTED from bauxite, `Al_(2)O_(3),2H_(2)O`. The extraction involves the following steps: (1) Purification (or concentration) of bauxite ore : (a) Baeyer's process : Red variety of bauxite containing iron oxide as the main impurity is purified by Baeyer's process. (b) Hall's process (Dry process) : Red bauxite is purified also by this method. (c) Serpeck's process : Bauxite ore containing silica as the chief impurity is purified by this process. Generally Hall's process is widely used for the purification of bauxite by fusing at high TEMPERATURE with `Na_(2)CO_(3)` in the presence of little lime, wvhen alumina, `Al_(2),O_(3)`dissolveformingsolubesodiumaluminate `NaAlO_(2)` .The filtrate of `NaAlO_(2)`is HEATED to 323-373k anda stram of `CO_(2)`is passedwhen `Al(OH_(3))` is precipitated. `Al(OH_(3))` pprecipitated is filtered, washed and ignited at 1473 K to obtain pure alumina, `Al_(2)O_(3)`. `underset("Bauxite")(Al_(2)O_(3)).2H_(2)O + Na_(2)CO_(3) overset(Delta)to2NaAlO_(2) + CO_(2) = 2H_(2)O` `2NaAlO_(2) + 3H_(2) O + CO_(2)overset(323 - 373 k) tounderset(ppt)(2Al)(OH)_(3) + Na_(2)CO_(3)` `2Al(OH)_(3) overset(1473k)to underset("pure alumina")(Al_(2)O_(3)) + 3H_(2)O` (2)Electrolysis of fused alumina (Hall and Heroult's process) : Purified alumina is dissolved in molten synthetic cryolite, `Na_(3)AlF_(6)`, and fused mixture is electrolysed with carbon electrodes. The molten electrolyte is covered with a layer of powdered coke to prevent the oxidation and loss of heat by radiation. 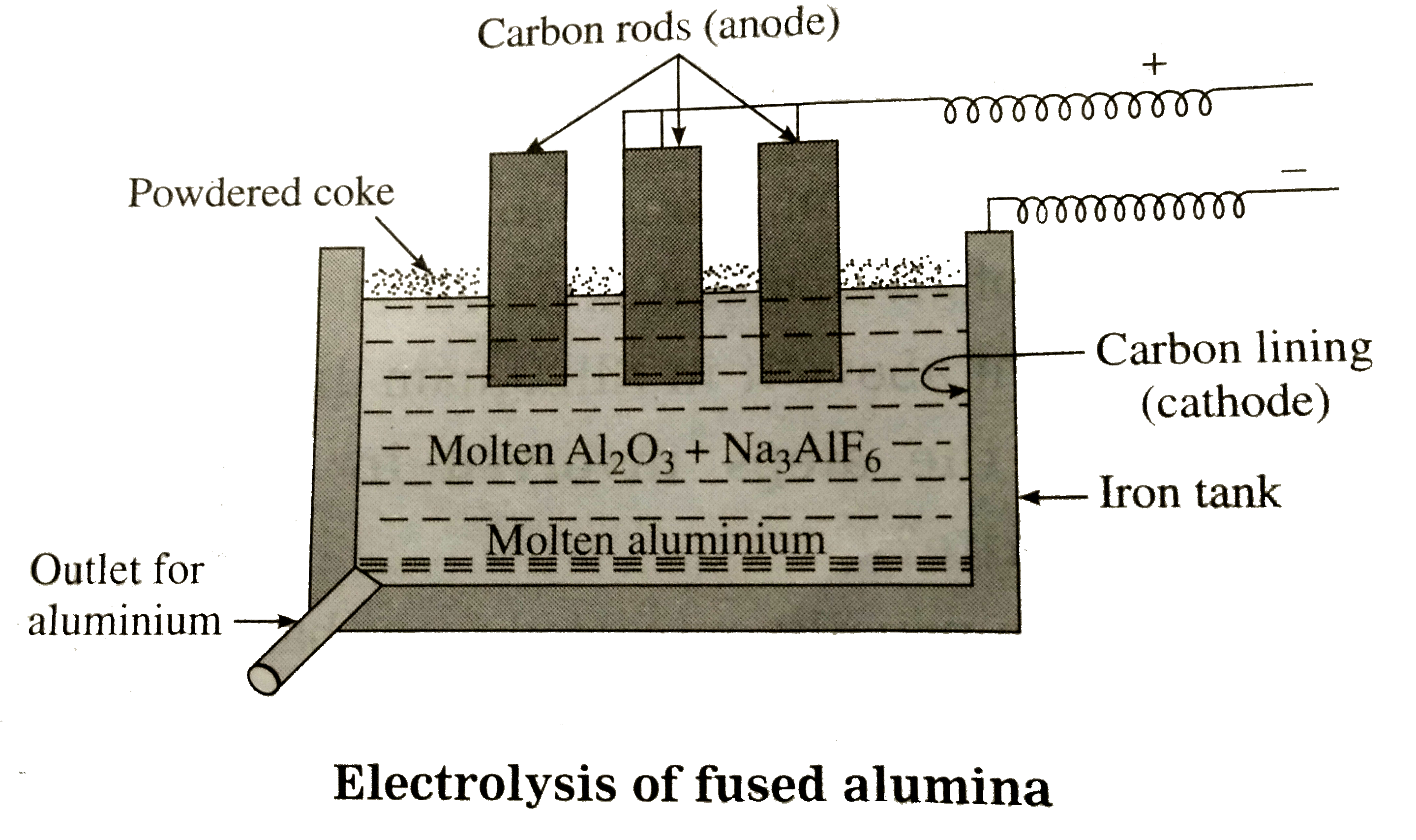 (3) Reactionsof electrolysis : `Na_(3)AIF_(6(l)) hArr 3NaF_((1)) + AIF_(3(1))` `AIF_(3(I)) hArr Al^(3+) + 3F^(-)` At cathode `: Al^(3+) + 3e^(-) to Al ""("reduce")` At anode `F^(-) to F + e^(-) ""(2F to F_(2(g)))` LIBERATED `F_(2)`reactswithaluminaand forms `AIF_(3)` . `2Al_(2) O_(3)+ 6F to4AIF_(3) + 3O_(2)` Theliberatedoxygenreacts with carbon of anodeformingCO and `CO_(2)` Moltenaluminiumcollectedat thebottomof the cellis priodicallyremoved. (4) Refiningof aluminium : Aluminiumis further purifiedby Hoop'selectrolyticprocess. The lowestlayeris of impuraluminiumand with carbon liningit formsan anode . Themiddlelayerconsistof a mixtureof cryolite, andNaF and server as anelectrolyt. Thetop layeris fo puremoltenaluminiumhavingcarbonelectrodes dippingin itwhichact as cathode. Duringelectrolysis `Al^(3+)`ions form middlelayerpass intotop layerandgetreducedat cathodeof formingpurealuminium. At thesame time,an equivlantamount of `Al^(3)` ions form thelowestlayer pass intomiddle layerbut impurities are leftin thelowestlayer. Thus,purealuminiumproudced , is periodically romovedform the top . |
|
Discussion
No Comment Found
Related InterviewSolutions
- Which of the following compounds is not cleaved by HI even at 525 K ?
- To a 25 mL H_(2)O_(2) solution excess of an acidified solution of potassium iodide was added. The iodine liberated required 20 " mL of " 0.3 N sodium thiosulphate solution Calculate the volume strength of H_(2)O_(2) solution.
- The suggested mechanism of a reaction is : (a) A+BhArrD("fast) "(b)A+Drarr2C("slow")Write the balanced equation of the reaction if its experimentally deduced rate equation is , rate k=[A]^(2)[B] Find the intermediate formed during the course of the reaction . Does the predicted rate law from the mechanism match the experimental rate law ?
- Which of these changes with time for a first-order reaction A Rate of reaction B . Rate constant C . Half-life
- What is the hybridisation of central atom in the product obtained along with hydrofluoric acid when complete hydrolysis of Xenon Hexa Fluoride takes place ?
- Which of the following amino acid forms sulphide bond in polypeptide
- Which of following pair is Diastereomers:
- What is the major product of the following reaction CH_3C-=C-CH_2-CH_3overset("1 mole of " Cl_2)to
- Which polymer is used in petrol tank linings ?
- Which of the following carbohydrates are branched polymer of glucose ?