Saved Bookmarks
| 1. |
what are thesalientfeature of Crystal FieldTheory(CFT) ? |
|
Answer» Solution :Bethe and vanVleckdeveloped Crystal FieldTheory (CFT) TOEXPLAIN variousproperties of coordinationcompounds . Thesalientfeatureof CFT are as follows: (1) In a complex , the CENTRAL metal atom or ions is surrounded by various ligands whichareeithernegativelycharged ions `(F^(-),Cl^(-), CN^(-))` etc.ornetural molecules `(H_(2)O,NH_(3))` en etc.) and the mostelectronegativeatom in themor ionand thesurroundingligandsact as pointtowardscentralmetal ions . (2) The metalatom or ions and the surroundingligandsact as pointcharges involvingpurely electrosn attractionbetweenthem. (3) (i)Thecentralmetal ion has five ( n -1) d degenerateorbitalsnamely `d_(xy),d_(yz),d_((x^(2)-y^(2)) and d_(z^(2))` (II)Whenthe ligandsapproch themetalion, dueto repulsiveforces, the degeneracyof d-orbitalsis destroyedand they split into two groups of differentenergy `t_(2g)` and `e_(g)`orbitals. This effect is calledcrystal filed splittingwhichdependsuponthe geometryof thecomplex. (iii)The d-oribtalslying in thedirections of ligands are affected to agereater extentwhile thoselyingin betweenthe ligandsare affectedto a lessextent. (iv) Due torepulsion, the orbitalsalongthe axesof ligands acquirehigherenergywhilethoselying in betweentheligands acquirelessenergy . (v) Hencerepulsion by ligands GIVE tow sets of split up orbitalsofmetal ion withdifferentenergies. 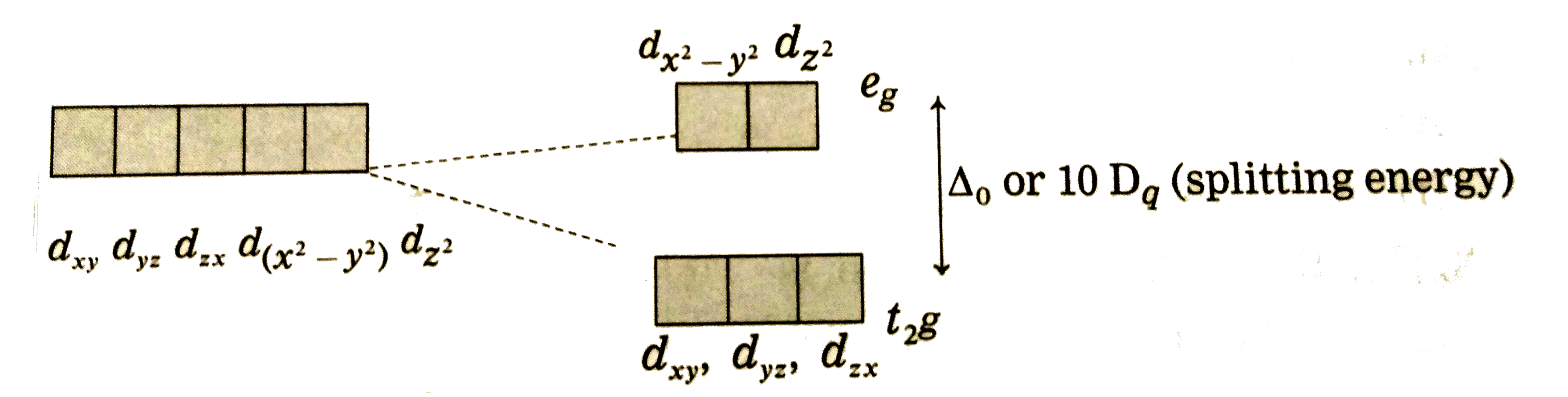 (vi)Theenergy differencebetweentwo sets of d-orbitalsaftersplittingby ligands is calledcrystal field splittingenergy(CFSE) andrespesented by `Delta_(0)`or by arbitrary term`10D_(q)`. Thevalue of `Delta` or `10Delta_(q)`dependsupon thegeometryof the complex. 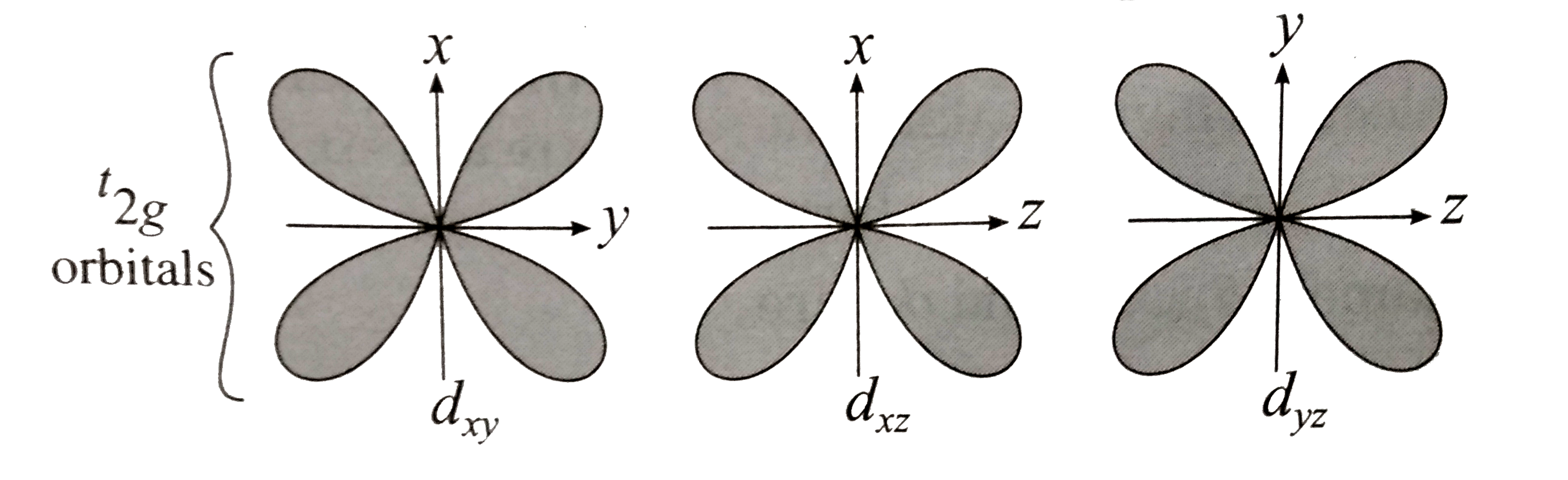 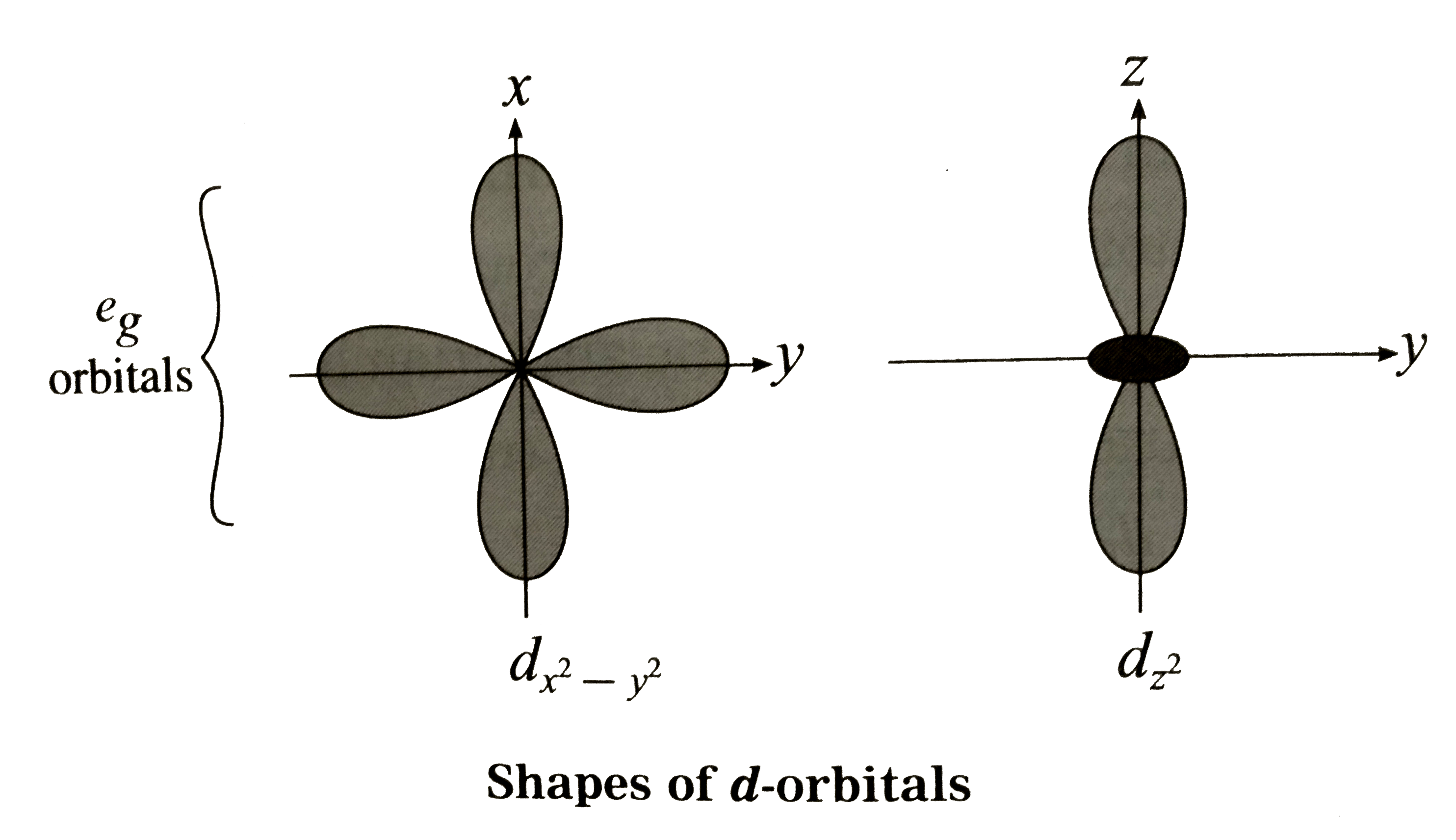 (4) Theelectrons of metalionoccupythe split d-orbitalsaccordingto Hund's ruleaufbauprincipleand thoseorbitalswithminimum repulsionand the firsthestawaysform theligands. (5) CFTdoes notaccountfor overlappingof orbitals of central metal ion and ligands , hencedoes notconsidercovalentnatureof thecomplex. (6) From thecrystal fieldstability ENERGY , the stability of thecomplexcan beknown. |
|
Discussion
No Comment Found
Related InterviewSolutions
- Which of the following compounds is not cleaved by HI even at 525 K ?
- To a 25 mL H_(2)O_(2) solution excess of an acidified solution of potassium iodide was added. The iodine liberated required 20 " mL of " 0.3 N sodium thiosulphate solution Calculate the volume strength of H_(2)O_(2) solution.
- The suggested mechanism of a reaction is : (a) A+BhArrD("fast) "(b)A+Drarr2C("slow")Write the balanced equation of the reaction if its experimentally deduced rate equation is , rate k=[A]^(2)[B] Find the intermediate formed during the course of the reaction . Does the predicted rate law from the mechanism match the experimental rate law ?
- Which of these changes with time for a first-order reaction A Rate of reaction B . Rate constant C . Half-life
- What is the hybridisation of central atom in the product obtained along with hydrofluoric acid when complete hydrolysis of Xenon Hexa Fluoride takes place ?
- Which of the following amino acid forms sulphide bond in polypeptide
- Which of following pair is Diastereomers:
- What is the major product of the following reaction CH_3C-=C-CH_2-CH_3overset("1 mole of " Cl_2)to
- Which polymer is used in petrol tank linings ?
- Which of the following carbohydrates are branched polymer of glucose ?