Saved Bookmarks
| 1. |
What is electrical conductance of a solution ? How is it measured experimentally ? |
|
Answer» Solution :The reciprocal of resistance (R ) is known as conductane (c ) `l/R=k xx(l)/(l//A)` `c=k xx(l)/(l//A)` `to` The conductance or the current conducting capacity of an electrolytic solution can be expressed as i) Specific conductance (k), ii) Molar conductivity `(^^_(m))` i) Specific conductance : The conductance of the solution enclosed between two parallel electrodes of unit area of cross section separated by unit distance is CALLED specific conductance (k). ii) Molar conductivity `(^^_(m)):` The conductivity of a volume of solution containing one gram molecular weiht of the electrolyte placed between two parallel electrodes separated by a distance of unit length of 1 meter is called molar conductivity `(^^_(m))` Relation between conductivity and molar conductivity : `^^_(m) =k/c,therefore c=` CONSTANT Measurement of electrical conductance : `to` The resistance of a metallic wire can be measured with a WHEATSTONE bridge. `to` In measuring the resistance of an electrolytic solution tow problems are identified. i) On PASSING direct current (DC) through the solution charges the composition of the solution due to electrolysis. ii) A solution cannot be connected to the mmeasuring bridge like a metallic wire. ` to` ii) A solution cannot be connected to the measuring bridge like a metallic wire. `to` First proble solved by using AC instead of DC. `to` Second problem solved by using specilly designed vessel called conductivity cell. 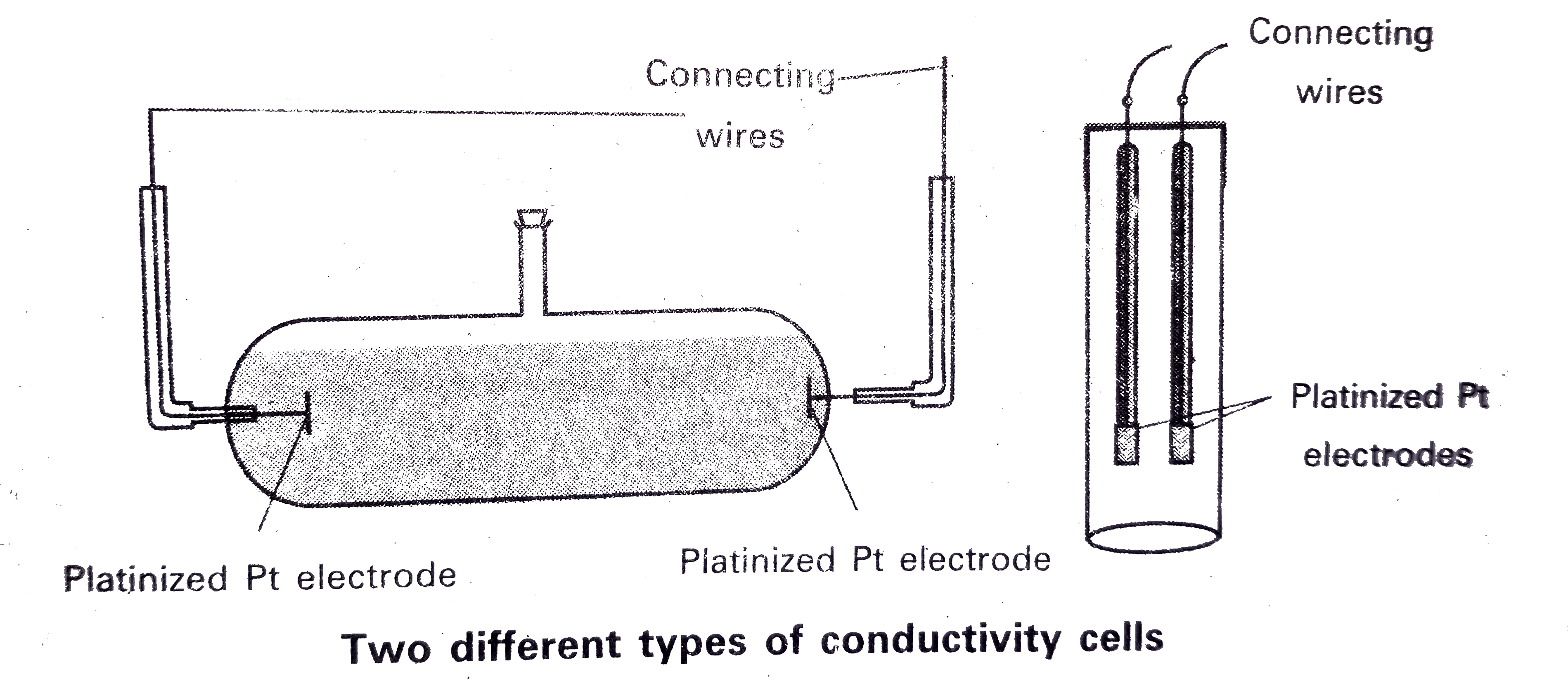 By using above cells, Resistance `R=(l)/(k xxA)` l = distance between electrodes : A = Area of cross section, k = conductivity `l/A` = cell constant `=G^(**)` `therefore G^(**)=l/A =Rxxk` The cell constant `(G^(**))` is determinded by measuring the resistance of the cell containing a solution whose conductivity is already known `to` Cell constant once determined, used for measuring the resistance (or) conductivity. `to` The set up for the measurement of the resistance is shown in following figure. Unknown resistance `R_(2) =(R_(1)R_(4))/(R_(3))` Conductivity of solution `(k) =("Cell constant " (G^(**)))/(R)` Molar conductivity `^^_((m))=k/c =(k (S CM ^(-1)))/(1000(Lm^(-3))xx"Molarity (moles//lit)")` 
|
|
Discussion
No Comment Found
Related InterviewSolutions
- Which of the following compounds is not cleaved by HI even at 525 K ?
- To a 25 mL H_(2)O_(2) solution excess of an acidified solution of potassium iodide was added. The iodine liberated required 20 " mL of " 0.3 N sodium thiosulphate solution Calculate the volume strength of H_(2)O_(2) solution.
- The suggested mechanism of a reaction is : (a) A+BhArrD("fast) "(b)A+Drarr2C("slow")Write the balanced equation of the reaction if its experimentally deduced rate equation is , rate k=[A]^(2)[B] Find the intermediate formed during the course of the reaction . Does the predicted rate law from the mechanism match the experimental rate law ?
- Which of these changes with time for a first-order reaction A Rate of reaction B . Rate constant C . Half-life
- What is the hybridisation of central atom in the product obtained along with hydrofluoric acid when complete hydrolysis of Xenon Hexa Fluoride takes place ?
- Which of the following amino acid forms sulphide bond in polypeptide
- Which of following pair is Diastereomers:
- What is the major product of the following reaction CH_3C-=C-CH_2-CH_3overset("1 mole of " Cl_2)to
- Which polymer is used in petrol tank linings ?
- Which of the following carbohydrates are branched polymer of glucose ?