Saved Bookmarks
| 1. |
What is molal depression constant (K_(f)) ? Derive equation relating K_(f) with molar mass of solute. |
|
Answer» SOLUTION :Decrease in freezing point of a solution prepared by dissolving one gram molar mass of non - volatile solute into one kilogram of solvent is called MOLAL depresion constant. Let `T_(f)^(0)` be the freezing point of PURE solvent and `T_(f)` be its freezing point when non - volatile solute is dissolved in it. The decrease in freezing point. `Delta T_(f)=T_(f)^(0)-T_(f)` is known as depression in freezing point. For dilute solution (ideal solution) is directly PROPORTIONAL to MOLALITY, m of the solution. Thus, `Delta T_(f)prop m` `Delta T_(f)=K_(f).m ""`....(i) The proportionality constant `K_(f)`, which depends on the nature of the solvent is known as Freezing Point Depression Constant or Molal Depression Constant or Cryoscopic Constant. The unit of `K_(f)` is K kg `mol^(-1)`. 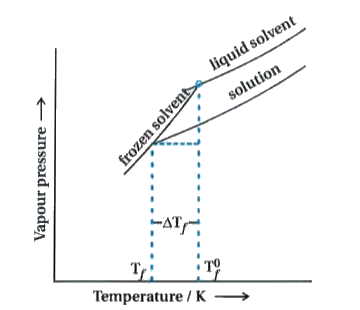 If `w_(2)` gram of the solute having molar mass as `M_(2)`, present in `w_(1)` gram of solvent, produces the depression in freezing point `Delta T_(f)` of the solvent then molality of the solute is given by the equation. `m=(w_(2)//M_(2))/(w_(1)//1000)` Substituting this value of molality in equation (i) we get, `Delta T_(f)=(K_(f)xx w_(2)//M_(2))/(w_(1)//1000)` `Delta T_(f)=(K_(f)xx w_(2)xx1000)/(w_(1)xx M_(2))` `M_(2)=(K_(f)xx w_(2)xx 1000)/(Delta T_(f)xx w_(1))` |
|
Discussion
No Comment Found
Related InterviewSolutions
- Which of the following compounds is not cleaved by HI even at 525 K ?
- To a 25 mL H_(2)O_(2) solution excess of an acidified solution of potassium iodide was added. The iodine liberated required 20 " mL of " 0.3 N sodium thiosulphate solution Calculate the volume strength of H_(2)O_(2) solution.
- The suggested mechanism of a reaction is : (a) A+BhArrD("fast) "(b)A+Drarr2C("slow")Write the balanced equation of the reaction if its experimentally deduced rate equation is , rate k=[A]^(2)[B] Find the intermediate formed during the course of the reaction . Does the predicted rate law from the mechanism match the experimental rate law ?
- Which of these changes with time for a first-order reaction A Rate of reaction B . Rate constant C . Half-life
- What is the hybridisation of central atom in the product obtained along with hydrofluoric acid when complete hydrolysis of Xenon Hexa Fluoride takes place ?
- Which of the following amino acid forms sulphide bond in polypeptide
- Which of following pair is Diastereomers:
- What is the major product of the following reaction CH_3C-=C-CH_2-CH_3overset("1 mole of " Cl_2)to
- Which polymer is used in petrol tank linings ?
- Which of the following carbohydrates are branched polymer of glucose ?