Saved Bookmarks
| 1. |
What is the (i) volume of gases evolved at STP and (ii)pH of solution on the electrolysis of 10L of the following solutions when 1F of electricity is passed : a. Aqueous solution of Na_(2)SO_(4) b. Aqueous solution of CH_(3)COONa c. Aqueous solution of HCOOK |
|
Answer» Solution :`a.` `i.` Since `E^(c-)._(red)` of `H_(2)gtE^(c-)._(red)` of `Na^(o+)` ion. and `E^(c-)._(o x i d)` of `H_(2)OgtE^(c-)._(o x i d e)` of `SO_(4)^(2-)` ion. So the reduction and oxidation of `H_(2)O` takes place to give `H_(2)(` CATHODE `)` and `O_(2)(` anode `)` , RESPECTIVELY. At cathode`:` `H_(2)O+e^(-) rarr overset(-)(O)H+(1)/(2)H_(2)(g)""(` Reduction `)` At anode `:` `H_(2)O rarr (1)/(2)O_(2)(g)+2H^(o+)+2e^(-)""(` Oxidation `)` First method At cathode `:` `1e^(-)+1F=(1)/(2)mol `of`H_(2)=(22.4)/(2)L` at `STP` `=11.2 L `of `H_(2)` At anode `:` `2e^(-)=2F=(1)/(2) mol `of `O_(2) =(22.4)/(2)L` at `STP` `:. 1F=(22.4)/(2x2) =5.6L` or `O_(2)` Total volume of gases at `STP=11.2+5.6=16.8L` Second method At cathode `:` `1F=1Eq O_(2)=(22.4)/(4)L=11.2L H_(2)` `(n` factor for `O_(2)=4)` Total volume `=(11.2 +5.6=16.8L)` `ii. pH` of solution Since `overset(c-)(O)H` ions are formed at cathode and `H^(o+)` ions are formed at anode. So they will remaing in the solution and will neutralize to give neutral solution. At cathode `:` `1F=1Eq OH` `[overset(-)(O)H]=("Equivalent")/("Volume in L")=(1)/(10)N` or `M` at anode `1F=1Eq of H^(o+)` or `2F=2 mol`of `H^(o+)` `[H^(o+)]=(1)/(10)N` or `M` Therefore, `0.1M overset(-)(O)H` ion will neutralize `0.1 M H ^(o+)` ion to give neutral solution with `pH=7` `b.` `i. CH_(3)COONa(ag)overset(El e ctrolysis)rarr CHCOO^(ddot(c-))+Na^(o+)` Since `E^(-)._(red )` of `H_(2)Ogt E^(c-)._(red)` of `Na^(o+)` ion,so reduction of water takes place at cathode to give `H_(2)(g)`. At cathode `:` `H_(2)O+e^(-) rarr overset(-)(O)H+(1)/(2)H_(2)uarr""(` Reduction `)` At anode `:` Kolbe's reaction `:` 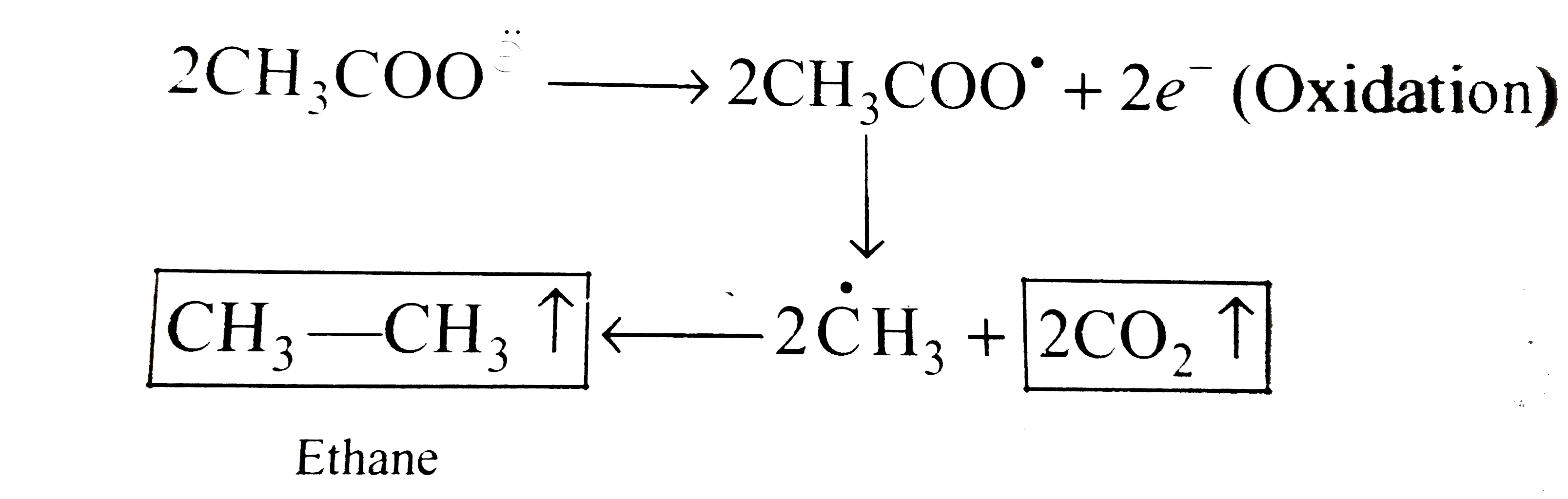 At anode, the oxidation of `CH_(3)COOoverset(ddot(c))` takes place to give `2 mol ` of `CO_(2)(g)` and ` mol ` of ethane `(C_(2)H_(6))` gas. At cathode `:` `1e^(-)=1F=(1)/(2) mol `of `H_(2)=(22.4)/(2)=11.2L` of `H_(2)` At anode `:` `2e^(-)=2F=(2mol ` of `CO_(2)+1 mol` of `C_(2)H_(6))` `:. 2F=3 mol ` of gases `1F=(3)/(2) mol` of gases `=(3)/(2)xx22.4 L` at `STP` `=33.6 L` at `STP` ltbtgt Total volume of gases at `STP =(11.2+33.6)` `=44.8L` `ii. pH` of solution `:` Solution will be basic due to the formation of `overset(-)(O)H` ions at cathode. `1e^(-)=1F=1Eq overset(-)(O)H` ion. `[overset(-)(O)H]=("Equivalent")/("Volume in L")=(1Eq)/(10L)=10^(-1)N` or `M` `:. pOH=1implies pH=14-1=13` `c.` `i. HCOOK (aq) overset(El ectrolysis)rarrHCOO^(ddot(c-))+K^(o+)` Since `E^(c-)._(red)` of `H_(2)OgtE^(-)._(red)` of `K^(o+)` ion, so reduction of `H_(2)O` takes place to give `H_(2)O(g)` . At cathode `:` `H_(2)O(g)+e^(-)rarr overset(-)(O)H+(1)/(2)H_(2)(g)""(`Reduction `)` At anode `:` Kolbe's reaction 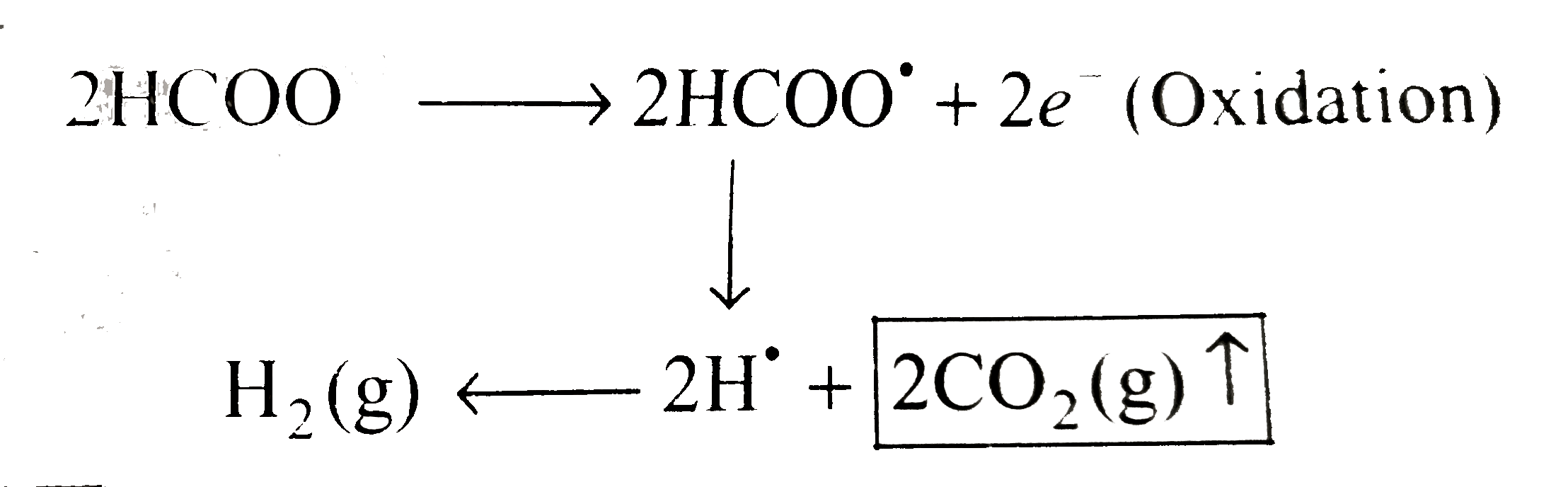 At anode, the oxidation of `HCOO^(ddo(c-))` takes place to give `1 mol `of `H_(2)O(g)`and `2 mol` of `CO_(2)(g)`. `1e^(-)=1F=(1)/(2) mol `of `H_(2)=(22.4)/(2)=11.2L` of `H_(2)` At anode `:` `2e^(-)=2F=(1 mol `of `H_(2)+2 mol` of `CO_(2))` `2F=3 mol` of gases `1F=(3)/(2) mol`of gases `=(3)/(2) xx 22.4 L `at `STP` `=33.6 L` at `STP` Total volume of gases at `STP =11.2 +33.6` =44.8 L` `ii. pH` of solution `=13[` PROCEED as in part `(b) (ii)` above `]` |
|
Discussion
No Comment Found
Related InterviewSolutions
- Which of the following compounds is not cleaved by HI even at 525 K ?
- To a 25 mL H_(2)O_(2) solution excess of an acidified solution of potassium iodide was added. The iodine liberated required 20 " mL of " 0.3 N sodium thiosulphate solution Calculate the volume strength of H_(2)O_(2) solution.
- The suggested mechanism of a reaction is : (a) A+BhArrD("fast) "(b)A+Drarr2C("slow")Write the balanced equation of the reaction if its experimentally deduced rate equation is , rate k=[A]^(2)[B] Find the intermediate formed during the course of the reaction . Does the predicted rate law from the mechanism match the experimental rate law ?
- Which of these changes with time for a first-order reaction A Rate of reaction B . Rate constant C . Half-life
- What is the hybridisation of central atom in the product obtained along with hydrofluoric acid when complete hydrolysis of Xenon Hexa Fluoride takes place ?
- Which of the following amino acid forms sulphide bond in polypeptide
- Which of following pair is Diastereomers:
- What is the major product of the following reaction CH_3C-=C-CH_2-CH_3overset("1 mole of " Cl_2)to
- Which polymer is used in petrol tank linings ?
- Which of the following carbohydrates are branched polymer of glucose ?