Saved Bookmarks
| 1. |
Which one of the following halogens forms only one acid? |
|
Answer» `Br_(2)` `F_(2)+H_(2)O overset(-40^(@)C) to HOF+HF` In `OF^(-)` ion, Fatom has no d-orbitals in its valence shell, and THUS it connot form pa-dn bonds with O-atom. Consequently, OF is not STABILIZED and therefore, HOF is highly unstable. Other halogens form a series of oxo acids such as `HXO, HXO_2, HXO_3 and HXO4`. Anions of these oxo acids are stabilized by pr-da bonding between full 2p-orbitals on oxygen atoms with empty-orbitals on halogen atoms, except fluorine. 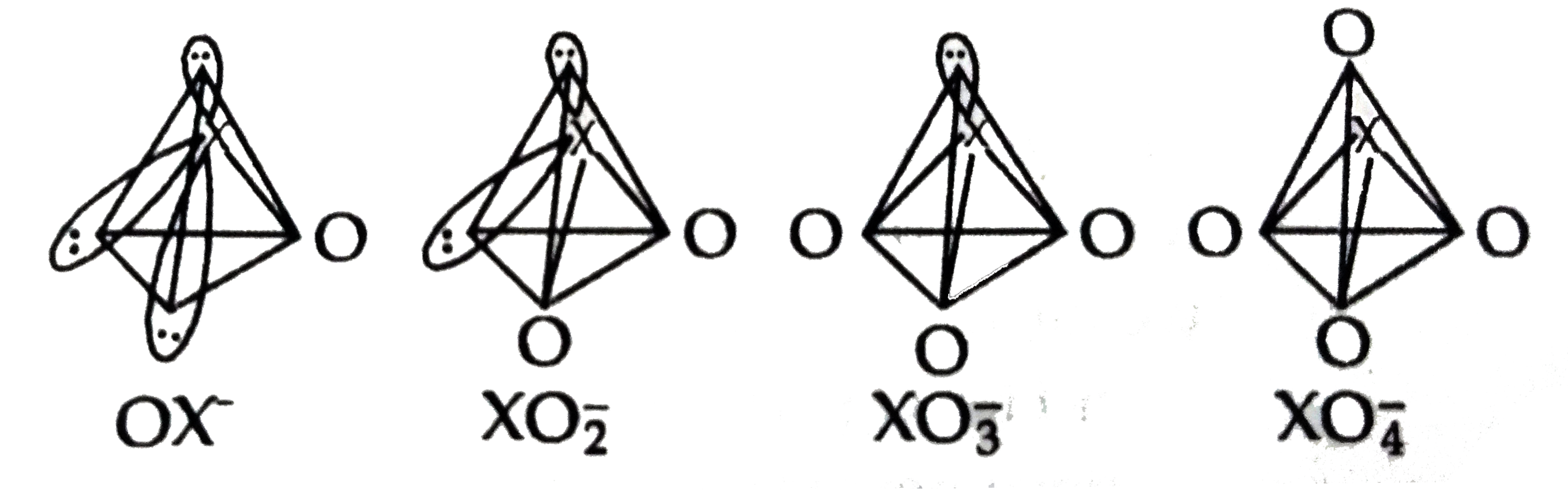
|
|
Discussion
No Comment Found
Related InterviewSolutions
- Which of the following compounds is not cleaved by HI even at 525 K ?
- To a 25 mL H_(2)O_(2) solution excess of an acidified solution of potassium iodide was added. The iodine liberated required 20 " mL of " 0.3 N sodium thiosulphate solution Calculate the volume strength of H_(2)O_(2) solution.
- The suggested mechanism of a reaction is : (a) A+BhArrD("fast) "(b)A+Drarr2C("slow")Write the balanced equation of the reaction if its experimentally deduced rate equation is , rate k=[A]^(2)[B] Find the intermediate formed during the course of the reaction . Does the predicted rate law from the mechanism match the experimental rate law ?
- Which of these changes with time for a first-order reaction A Rate of reaction B . Rate constant C . Half-life
- What is the hybridisation of central atom in the product obtained along with hydrofluoric acid when complete hydrolysis of Xenon Hexa Fluoride takes place ?
- Which of the following amino acid forms sulphide bond in polypeptide
- Which of following pair is Diastereomers:
- What is the major product of the following reaction CH_3C-=C-CH_2-CH_3overset("1 mole of " Cl_2)to
- Which polymer is used in petrol tank linings ?
- Which of the following carbohydrates are branched polymer of glucose ?