Saved Bookmarks
| 1. |
Write the reactions involved in extraction of silver from its ore by leaching process. Derive the equation : W = -P_("ext"*DeltaV) A unit cell of iron crystal has edge length 288 pm and density7*86 "g"cm^(-3) .Find the number of atoms per unit cell and type of the crystal lattice . Given : Molar ,mass of iron = 56 g"mol"^(-1) avogadro's number N_(A) = 6*022 xx 10^(23) |
Answer» Solution :(a) STEP -1 : The METAL is leached with doilute solution of NaCN or KCN in the presence of air `(O_(2))` , so that a soluble complex of the metal is obtained . 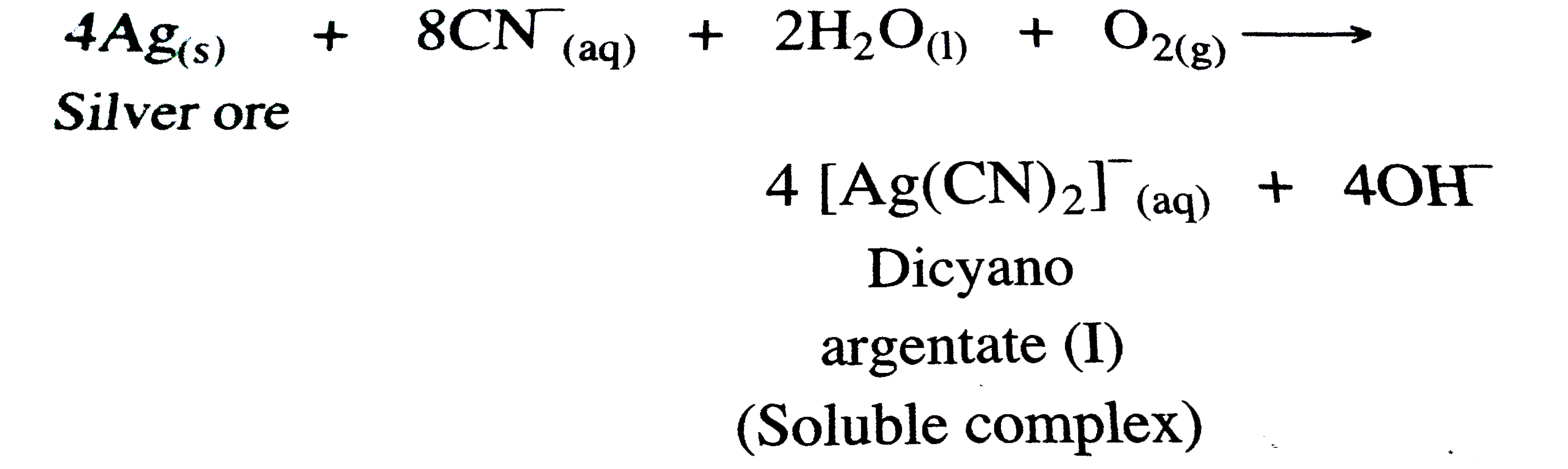 , ,STEP - 2 : From TBE soluable complex `[M(CN_(2))]^(-)` , the metal Ag is precipitated by more electropositive or active metals like Zn . 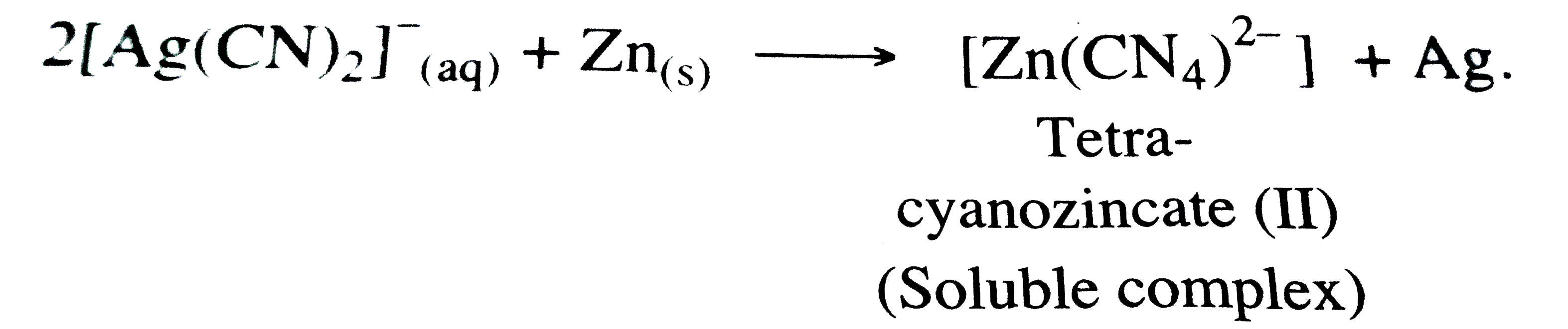 (b) Consider a certain amount of an IDEAL gas enclosed in an ideal cylinder fitted with ,massless , frictionless rigid movable piston at pressure P, occupying volume `V_(1)` at Temperature T . As the gas expands , it pushes the piston upward through a distance d against sternal force F , pushing the surroundings . The WORK done by the gas is, `W = "Opposing force" xx "Distance"` `W = -Fxxd` -ve sign indicates the lowering of energy of the system during expansion . If a is the - section area of the cylinder or piston , then , ` W = -F/a xx d xx a` Now the pressure is, ` P _("ex") = F/a` While volume change is , ` Delta V = d xxa` ` :. W = -P _("ex") xx Delta V` If during the expansion , the volume changes from `V_(1) and V_(2)` then , ` Delta V = V_(2) - V_(1)` ` :. W = -P _("ex")(V_(2)-V_(1))` (c )GIVEN that : 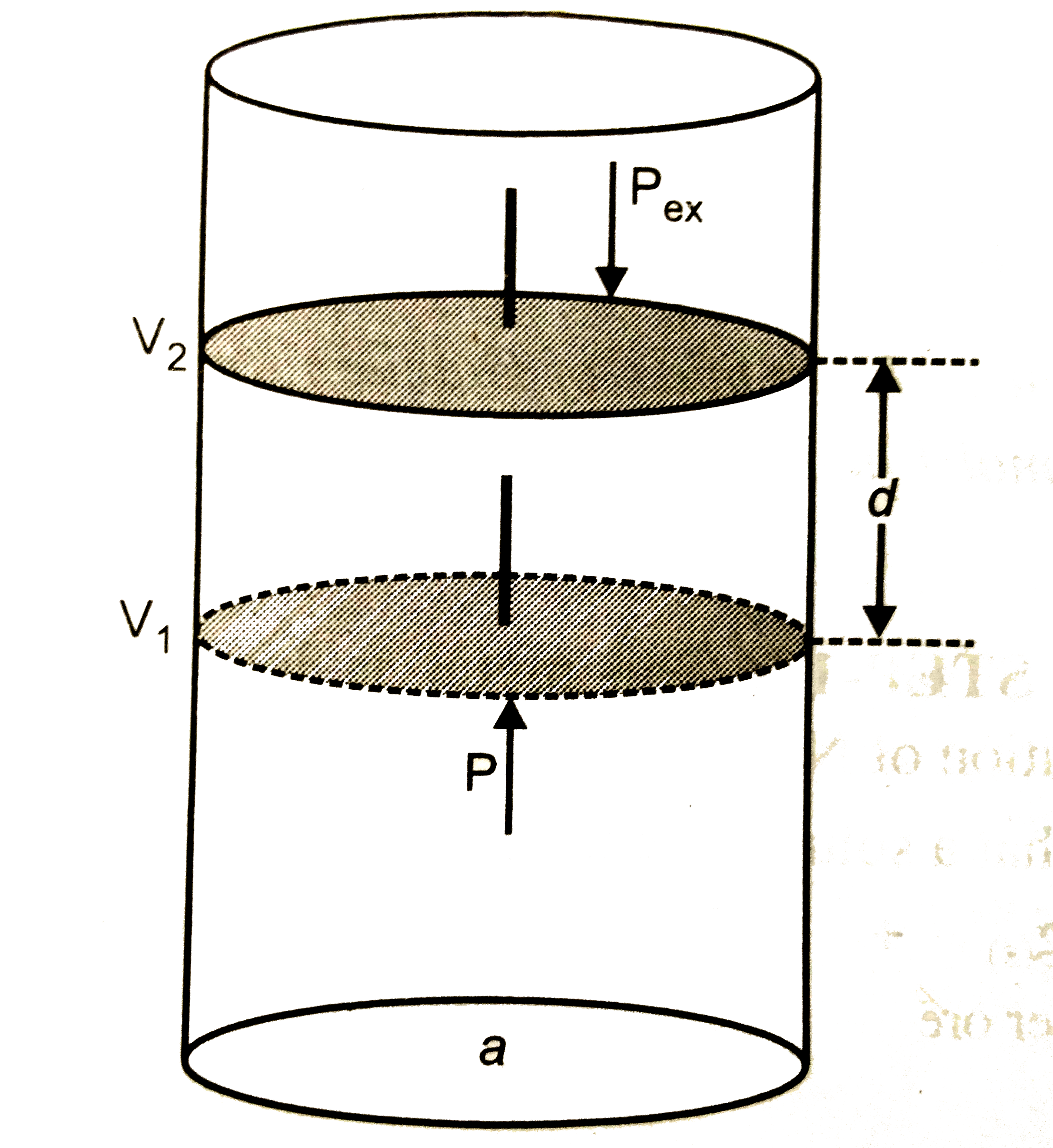 ` d = 7*86 g//cm^(3)` ` a = 288 ` ppm or ` = 2*88 xx 10^(-8)` cm ` N_(A) = 6*022 xx10^(23) "mol"^(-1)` Molar mass = 56 g/mol ` d = (ZxxM)/(a^(3) xxN_(A))` ` Z = (d xx a^(3) xx N_(A))/M` ` = (7*86 xx(2*88 xx10^(-8))^(3)xx6*022 xx10^(23))/56` ` = 2*01 ~~ 2` Hence, the unit cell of crystal lattice is of bcc type. |
|
Discussion
No Comment Found
Related InterviewSolutions
- Which of the following compounds is not cleaved by HI even at 525 K ?
- To a 25 mL H_(2)O_(2) solution excess of an acidified solution of potassium iodide was added. The iodine liberated required 20 " mL of " 0.3 N sodium thiosulphate solution Calculate the volume strength of H_(2)O_(2) solution.
- The suggested mechanism of a reaction is : (a) A+BhArrD("fast) "(b)A+Drarr2C("slow")Write the balanced equation of the reaction if its experimentally deduced rate equation is , rate k=[A]^(2)[B] Find the intermediate formed during the course of the reaction . Does the predicted rate law from the mechanism match the experimental rate law ?
- Which of these changes with time for a first-order reaction A Rate of reaction B . Rate constant C . Half-life
- What is the hybridisation of central atom in the product obtained along with hydrofluoric acid when complete hydrolysis of Xenon Hexa Fluoride takes place ?
- Which of the following amino acid forms sulphide bond in polypeptide
- Which of following pair is Diastereomers:
- What is the major product of the following reaction CH_3C-=C-CH_2-CH_3overset("1 mole of " Cl_2)to
- Which polymer is used in petrol tank linings ?
- Which of the following carbohydrates are branched polymer of glucose ?