InterviewSolution
This section includes InterviewSolutions, each offering curated multiple-choice questions to sharpen your knowledge and support exam preparation. Choose a topic below to get started.
| 7052. |
The turbidity of a polymer solution measures : |
|
Answer» a LIGHT ABSORBED by solution |
|
| 7053. |
Which of the following pairs is an example of position isomerism |
|
Answer» `CH_(3)-CH_(2)-CH_(2)-CH_(3)` and `CH_(3)-underset(CH_(3))underset(|)(CH)-CH_(3)` |
|
| 7054. |
Which of the following are addition homopolymers ? |
|
Answer» Teflon |
|
| 7055. |
What is the chemical composition of malachite? |
|
Answer» `CUO. CuCO_(3)` |
|
| 7056. |
When the temperature is lowered and pressure is raised, the adsorption of a gas on a solid |
|
Answer» Decreases |
|
| 7057. |
X(C_8H_14) by ozonolysis forms Y[C_8H_14O_2].Y on reaction with NaOI followed by acidification gives CHI_3 and compound Z on strong heating froms W. {:("Column-I","Column-II"),((A)"Compound X","(p)Bayer's Test"),((B)"Compound Y",(q)NaHCO_3),(( C)"Compound Z","(r)2, 4-DNP"),((D)"Compound W","(s)Iodoform Test"),(,"(t)Na Metal"):} |
|
Answer» OZONOLYSIS product `Y[C_8H_14O_2]` has 8 C ATOMS indicates (X) is cyclic compound with one double BOND. Thus Ais symmetrical CYCLOHEXENE. 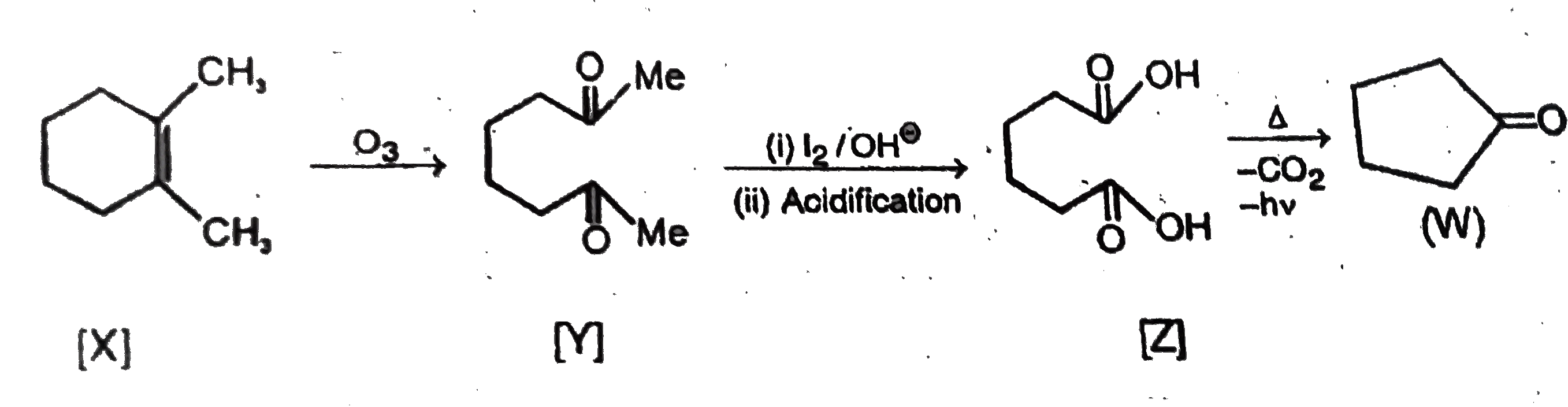
|
|
| 7058. |
What is the co-ordination entity formed when excess of aqueous KCN is added to an aqueous solution of copper sulphate ? Why is that no precipitate of copper sulphide is obtained when H_2 S(g) is passed through this solution ? |
|
Answer» Solution :`K_2 [Cu(CN)_4]` is formed when excess of aqueous KCN is added to an aqueous solution of `CuSO_4` . `underset("SULPHATE soluble")underset("Copper")(CuSO_4(aq)) + underset("CYANIDE")underset("Potassium")(4KCN(aq)) to underset("tetracyanocuprate (II)")underset("Potassium")(K_2 [Cu(CN)_4]) + K_2 SO_4 (aq)` As `CN^-` ions are strong ligands the complex is very stable. It is not replaced by `S^(2-)` ions when `H_2 S` gas is passed through the solution and thus no precipitate of CUS is obtained. |
|
| 7059. |
Which of the following compounds does not react with NaOH ? |
|
Answer» `CH_(3)COOH` `CH_3CONH_2overset(NaOH)rarrCH_3COONa+NH_3` `C_6H_5OH overset(NaOH)rarrC_6H_5ONa+H_2O` But alcohos are weaker than water and ract with ALKALI METALS to form alkoxids and not with ALKALIES. |
|
| 7060. |
Which one of the following is used in the extraction of alumininum by electronlytic process |
| Answer» Answer :A | |
| 7061. |
Which of the following compounds has meso isomer? |
|
Answer» `CH_(3)CHNH_(2)CONH_(3)` |
|
| 7062. |
Write one reaction using alkaline KMnO_(4). |
| Answer» Solution :`underset("Ethylene")(overset(CH_(2))overset(||)(CH_(2))) +H_(2)O+(O) underset("(BAEYER's REAGENT)")overset("Alkaline"KMnO_(4))(RARR) underset("Ethylene glycol")(overset(CH_(2)OH)overset(|)(CH_(2)OH))` | |
| 7063. |
Which of the following pair of compounds give same gaseous product on decomposition |
|
Answer» `KMnO_(4), K_(2)Cr_(2)O_(7)` |
|
| 7064. |
When chlorine gas is passed through and aqueous solution of potassium iodide containing strach, colour or the solution becomes............... . |
| Answer» SOLUTION :The `I_(2)` liberated from `KI (2 KI + Cl_(2) rarr 2 KCL + I_(2))` TURNS starch solution BLUE. | |
| 7066. |
Why 3^@-butyl benzene does not give benzoic acid on oxidation with hot alkaline KMnO_4? |
| Answer» Solution :Alkyl BENZENE is oxidised to BENZOIC acid with HOT alkaline `KMnO_4` when it has at least one BENZYLIC HYDROGEN. Since `3^0`-butyl benzene has no benzylic hydrogen atoms it is not oxidised by hot alkaline `KMnO_4`. | |
| 7067. |
Which of the following complex ion has tetrahedral arrangement ? |
|
Answer» `[AuCl_(4)]^(-)` |
|
| 7068. |
Which of the following is/are wrong? |
|
Answer» PhCO-`NH_(2)` gives BAD SMELLING substance with `CHCl_(3)`KOH |
|
| 7069. |
The value of Henry's constant K_(H) is …….. |
|
Answer» Greater for gases with higher SOLUBILITY. |
|
| 7070. |
Total number of unpaired electrons present in Co^(3+) (Atomic number = 27) is |
|
Answer» 2 |
|
| 7071. |
Which of the following metal(s) in liquid NH_(3) with low conc. Is not paramagnetic? |
|
Answer» `CS` |
|
| 7072. |
Which of the following forms is an ideal solution? |
|
Answer» ETHYL BROMIDE + ethyhl iondide |
|
| 7073. |
Which enzyme hydrolyses triglyceride to fatty acids and glycerol |
|
Answer» Amylase |
|
| 7074. |
The specific rate constant of a first order reaction depends on the |
|
Answer» CONCENTRATION of the reactant |
|
| 7075. |
Which of the followingcompounds in NOT prepared by the action of alcoholic NH_(3) on alkyl halide ? |
|
Answer» `CH_( 3)NH_(2)` |
|
| 7076. |
Two elements A and B form compounds having formula AB_(2) and AB_(4). When dissolved in 20 g of benzene (C_(6)H_(6)), 1 g of AB_(2) lowers the freezing point by 2.3 K whereas 1.0 g of AB_(4) lowers it by 1.3 K. The molar depression constant for benzene is 5.1 K kg mol^(-1). Calculate atomic masses of A and B. |
|
Answer» Solution :We know that, `M_(2)=(1000xx w_(2)xx K_(f))/(Delta T_(f)xx w_(1))` Then, `M_(AB_(2))=(1000xx1xx5.1)/(2.3xx20)=110.87g mol^(-1)` `M_(AB_(4))=(1000xx1xx5.1)/(1.3xx20)=196.15 g mol^(-1)` Now, we have molar masses of `AB_(2)` as `110.87 g mol^(-1)`and `196.15 g mol^(-1)` RESPECTIVELY. Let the atomic masses of A and B x and y respectively. Now, we can write : `x + 2y=110.87 ""`.....(i) `x + 4y=196.15 ""`....(ii) SUBTRACTING EQUATION (i) from (ii), we have `2y=85.28` `therefore y=42.64` Putting the value of y in equation (1), we have `x + 2xx42.64 = 110.87` `therefore x = 85.28` Hence, the atomic masses of A and B are 25.59 u and 42.64 u respectively. |
|
| 7077. |
Which of the following is an example of endothermic reaction |
|
Answer» `C_(2)H_(2)+2H_(2)rarrC_(2)H_(6),DeltaE=-314.0 kJ` `N_(2)+(1)/(2)O_(2)rarrN_(2)O DeltaH=+ve` `N_(2)+O_(2)rarr2NO DeltaH=+ve` |
|
| 7078. |
What is Browanian movement of colloidal solution? What is its cause ? |
| Answer» | |
| 7079. |
The Simplest formula of the compound containing 50% X (Atomic mass 10 amu) and 50% Y(Atomic mass 20amu) is: |
|
Answer» `XY_(2)` Formula=`X_(2)Y` |
|
| 7080. |
Which of the followingis basically responsiblefor acidicnatureof phenols ? |
|
Answer» Displacement of lone PAIR form oxygen atom. |
|
| 7081. |
What IUPAC name would you give to the following compound? H_(3)C-overset(O)overset(||)C-CH_(2)-overset(Cl)overset(|)CH-CH_(3) |
| Answer» SOLUTION :4 - Chloropental -2- ONE. | |
| 7082. |
Which one of the following is correctly matched ? |
|
Answer» |
|
| 7083. |
What IUPAC name would you give to the following compound ? H_(3)C-overset(O)overset(||)C-CH_(2)-overset(Cl)overset(|)CH-CH_(3) |
| Answer» SOLUTION :4 - Chloropental -2- ONE. | |
| 7084. |
Which of the following comments are true for a dry cell - |
|
Answer» a ZN can (or CONTAINER) ACTS as the anode `MnO_(2)` and carbon black remain in contact with a Zn can |
|
| 7085. |
Which of the following statement is correct regarding following species? (i) Cl overset(E.A.)to Cl^(-) (ii) Cl^(-) overset(I.E.)to Cl (iii) Cl overset (I.E.) to Cl^+ (iv) Cl^(+) overset(I.E.)to Cl^(2+) |
|
Answer» |I.E. of PROCESS (II)|=|E.A. of process (i)| |
|
| 7086. |
Which of the following will exhibit cis-trans isomerism? |
|
Answer» `CH_(2)Br-CH_(2)Br` |
|
| 7087. |
Which of the following is absent in RNA ? |
|
Answer» THYMINE |
|
| 7088. |
When SO_2 gas is passed through cupric chloride solution: |
|
Answer» The solution becomes colourless |
|
| 7089. |
Which of the following statement is correct about halobenzene? |
|
Answer» lt are more reactive than HALOALKANE |
|
| 7090. |
Why all adsorptions are exothermic in nature ? |
| Answer» SOLUTION :ADSORPTION results because of attraction between the constituents of adsorbate and ADSORBENT. Since energy is ALWAYS released during attraction, it is an EXOTHERMIC process. | |
| 7091. |
Which one of the following is not feasible? |
|
Answer» `ZN(s)+CU^(2+)(aq)toCu(s)+Zn^(2+)(aq)` |
|
| 7092. |
which of the following option is correct? |
|
Answer» In living organisms, circulation of `^14C`from atmosphere is high so the carbon content is content in organism |
|
| 7093. |
What is freezing point of a liquid ? |
| Answer» Solution :Freezing point is the tempreature at which the vapour pressure of the liquid and the solid state BECOME identical i.e both solid and liquid states COEXIST. The liquid and solid are in EQUILIBRIUM state . The freezing point of a solution is LESS than the freezing point of a pure solvent . | |
| 7094. |
Which of the following has greatest number of atoms? |
|
Answer» 1g of butane `(C_(4)H_(10))` |
|
| 7095. |
The type of colloidal dispresino obtained when egg protein is mixed with water is called …………(multimolecular or macromolecular or associated colloid). |
| Answer» SOLUTION :MACROMOLECULAR | |
| 7096. |
Which gas is filled in electric bulbs/tubes: |
|
Answer» `O_2` |
|
| 7097. |
What is smelting? Explain with an example. |
|
Answer» Solution :(1) Smelting : The PROCESS of extraction of a metal from itsore by heating and melting at high temperature is called smelting. (2) The ores of less electropositive metals after concentration androasting are REDUCED by strongly heating with powerful reducing agents like Na, Mg, Al in the blast furnace . `Cr_(2)O_(3) + overset(Delta)to2Cr + Al_(2)O_(3)` (3) During smelting, flux is added to convert INFUSIBLE impurities (gangue) into a slag. `FeO_((S)) underset(flux)(SinO_(2))overset(Delta)to underset("slag")(FeSiO_(3))` (4)During smelting autoreduction also takes place giving molten metal, `2Cu_(2)O +Cu_(2)S to 6Cu_((l)) +SO_(2)` |
|
| 7098. |
Which among the followings is most basic in aqueous solution |
|
Answer» PRIMARY methylamine |
|
| 7099. |
What are the two types ofbuffer? Give an example for each. |
|
Answer» Solution :(i) ACIDIC buffer solution :a solution containing a weak ACID and its salt. Example. Solution containing ACETIC acid and sodium acetate (ii) BASIC buffer solution :a solution containing a weak base and its salt. Example : Solution containing `NH_4OH and NH_4 CI`. |
|
| 7100. |
The shape of IF_(6)^(-) is : |
|
Answer» Trigonally distorted octahedron 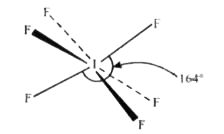
|
|