Explore topic-wise InterviewSolutions in .
This section includes InterviewSolutions, each offering curated multiple-choice questions to sharpen your knowledge and support exam preparation. Choose a topic below to get started.
| 22901. |
Which of the following gas is used in oxyacetylene welding |
|
Answer» `O_2` |
|
| 22902. |
Which of the following will be products of following reaction ? |
|
Answer»
|
|
| 22903. |
Write one chemical equation to show that SO_2 acts as areducing agent. |
|
Answer» SOLUTION :`SO_2` REDUCES `KMnO_4` to `MnSO_4` `2KMnO_4 + 2H_2O + 5SO_2 RARR K_2SO_4 + 2MnSO_4 + 2H_2SO_4` |
|
| 22904. |
Which of the following haldies has theleast dipole moment? |
|
Answer» `1,2-` Dichlorobenzane 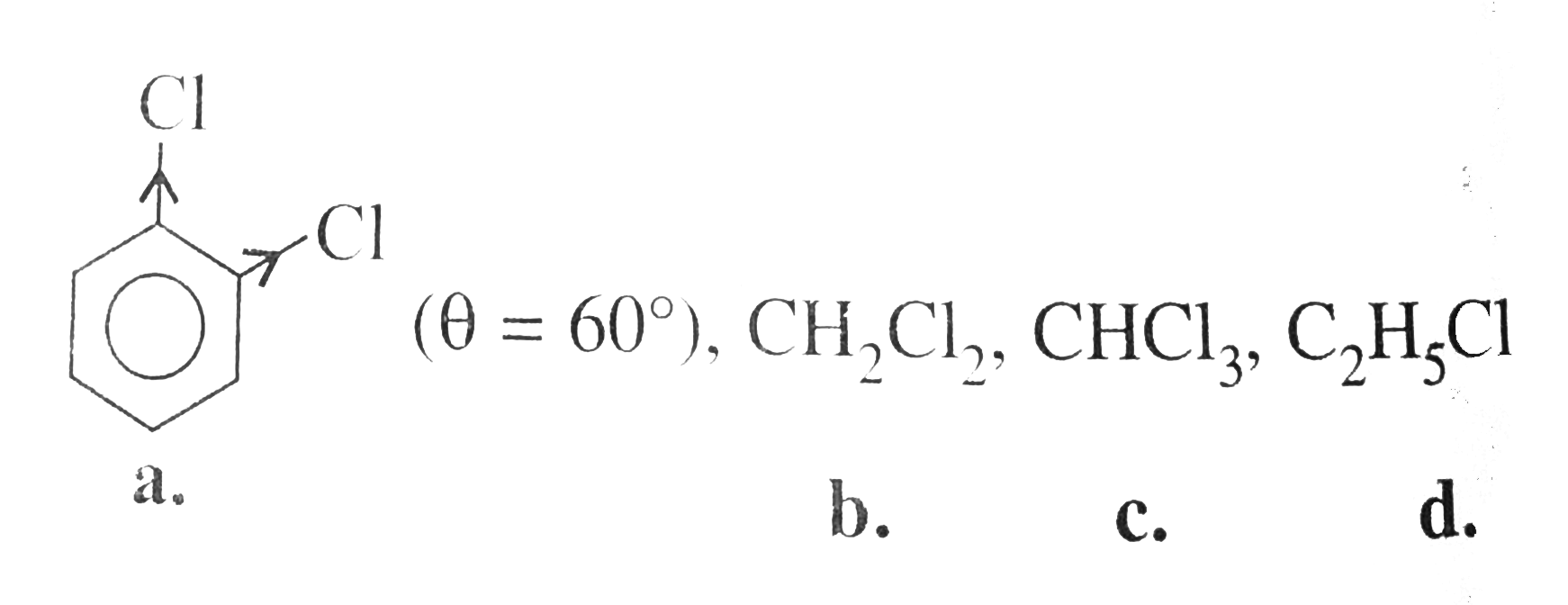 `MU` ORDER is: `(a) GT (d) gt (B) gt (C)` |
|
| 22905. |
Write the chemical reactions associated with the 'borax bead test' of cobalt (II) oxide. |
| Answer» | |
| 22907. |
Which of the following are less basic than H_(2)C=overset(* *)NH? |
|
Answer» `CH_(3)-C-=OVERSET(* *)N` 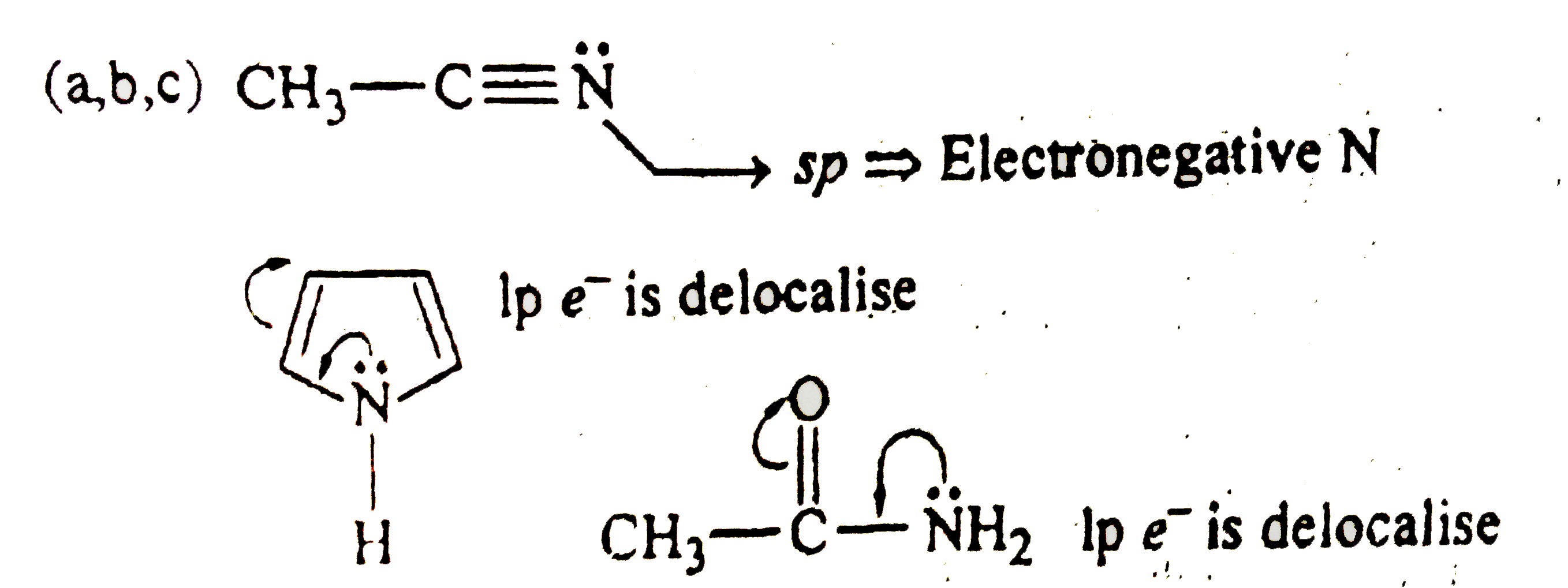
|
|
| 22908. |
Write the overall reaction that occurs during use (discharging) of nickel- cadmium cell. Is it a primary or a secondary cell? Mention its one merit over the lead storage cell. |
|
Answer» Solution :(i) Nickel-cadmium storage cell: It is another rechargeable cell. It consists of cadmium anode and the CATHODE is made of a metal grid containing nickel (IV) oxide. These are immersed in KOH solution. The overall reactions occuring: Anode: `Cd(s)+2OH^(-) (aq) to Cd(OH)_2(s)+2e^(-)` Cathode: `NiO_2(s)+2H_2O(l)+2e^(-)to NI(OH)_2(s)+2OH^(-) (aq)` Net reaction `Cd(s)+NiO_2(s)+2H_2O(l)to Cd(OH)_2(s)+Ni(OH)_2(s)` discharging Ni-Cd cell is : `Cd(s)+2NI(OH)_3(s)to CdO(s)+2Ni(OH)_2(s)+H_2O(l)` (ii) Primary/Secondary: It is a secondary cell. (iii) Merit over lead storage cell: As is evident, there are no gaseous products, the products formed. adhere to the electrodes and can be reconverted by recharging process. |
|
| 22909. |
Which of the following gives a tertiary alcohol when treated with Grignard reagent ? |
|
Answer» `H-OVERSET(O)overset("||")C-H` 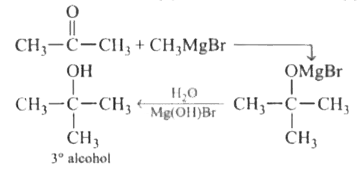
|
|
| 22910. |
What happens when a Cu rod is dipped in Fe_(2)SO_(4) solution ? |
|
Answer» FE PPT. |
|
| 22911. |
The work of catalyst depends on |
| Answer» Solution :volume of particles | |
| 22912. |
Which one of the following given d-block elements has the lowest atomic radii? |
|
Answer» Cu |
|
| 22913. |
Which one has the highest boiling point ? |
|
Answer» He |
|
| 22914. |
Whatare hormones ? Mentiontheir function. Namesome hormones. |
|
Answer» (ii) The majorendogrineglandsare thepitutiary , pineal , thymusthyroid , adernalglandsand PANCREASE. In additionalmen proudces hormones in theirtestes and women produceshormones in their ovary . |
|
| 22915. |
What are interstitial compounds? |
|
Answer» Solution :(i) An interstitial compound or alloy is a compound that is formed when small atoms like hydrogen, boron, carbon or nitrogen are trapped in the interstitial HOLES in a metal lattice. (ii) They are usually non-stoichiometric compounds. (iii) Transition metals form a number of interstitial compounds such as `Tic, ZrH_(1.92), Mn_(4)N` etc. (iv) The elements that occupy the metal lattice PROVIDE them new properties. 1. They are hard and show electrical and thermal conductivity 2. They have high melting points HIGHER than those of pure metals 3. Transition metal hydrides are USED as powerful reducing agents 4. Metallic carbides are chemically inert. |
|
| 22916. |
What is calcination? |
| Answer» SOLUTION :Calcination is a PROCESS of HEATING of the concentrated ore below its melting point with limited SUPPLY of ir , or in the absence of air. | |
| 22917. |
Why are adsorbate particles attracted and retained on the surface of adsorbent ? |
| Answer» SOLUTION :The unbalanced forced of the ADSORBENT are responsible for ATTRACTING adsorbate PARTICLES at adsorbent SURFACE . | |
| 22918. |
Which of the following form micellies in aqueous solution above critical concentration |
|
Answer» Glucose |
|
| 22919. |
Which of the following liquid pairs shows a positive deviation from Raoults Law ? |
|
Answer» `H_2O -HCL` |
|
| 22920. |
Which of the following enhances leathering property of soap? |
|
Answer» SODIUM carbonate |
|
| 22921. |
Which of the following is a non electrolytic colloidal sol |
| Answer» Answer :A | |
| 22922. |
The vapour pressure of pure liquids A and B are 450 and 700 mm Hg respectively, at 350 K. Find out the composition of the liquid mixture if total vapour pressure is 600 mm Hg. Also find the composition of the vapour phase. |
|
Answer» SOLUTION :Composition of Liquid mixture : Vapour pressure of pure liquid A `(p_(A)^(0))=450` mm Vapour presure of pure liquid B `(p_(B)^(0))=450 mm` Total vapour pressure of solution (P) = 600 mm According to Roult.s law : `p = p_(A)^(0)chi_(A)+p_(B)^(0)chi_(B)` `p=p_(A)^(0).chi_(A)+p_(B)^(0)(1-chi_(A))` `p=450.chi_(A)+700(1-chi_(A))` `600=450 chi_(A)+700-700 chi_(A)` `600-700=-250 chi_(A)` `chi_(A)=0.40` So, mole fraction of B = 0.60 Composition of vapour PHASE : `p_(A)=p_(A)^(0).chi_(A)=450xx0.40=180 mm` `p_(B)=p_(B)^(0).chi_(B)=700xx0.60=420 mm` Mole fraction of A in vapour `= (p_(A))/(p_(A)+p_(B))` `= (180)/(180+420)=0.30` So, mole fraction of B is = 0.70. |
|
| 22923. |
When a chemical bond is formed, the energy of the system: |
|
Answer» INCREASES |
|
| 22924. |
Which of the following amino acids is not optically active? |
|
Answer» Alanine |
|
| 22925. |
Which of the following is an example of basic dye? |
|
Answer» Alizarine  Malachite. |
|
| 22926. |
Which of the following statement is /are correct- |
|
Answer»

|
|
| 22928. |
Write the structures of A, B, C and D in the following reactions : |
| Answer» Solution :`underset("Acetic acid")underset()(CH_(3)COOH)OVERSET(PCl_(3))rarrunderset([A])underset("Acetyl chloride")underset()(CH_(3)COCl)overset(H_(2)//Pd//BaSO_(4))rarrunderset([B])underset("ACETALDEHYDE")underset()(CH_(3)CHO)overset(CH_(3)MgBr)underset(H_(3)O^(+))rarrunderset([C])underset("Isopropyl alcohol")underset()((CH_(3))_(2)CHOH)` | |
| 22929. |
Which of the following is a positively charged sol ? |
|
Answer» `FE(OH)_3` |
|
| 22930. |
Write the Nernst equation. |
|
Answer» Solution :`E_("cell")=E_("cell")^(@)-(RT)/(NF)In([C]^(L)[D]^(m))/([A]^(x)[B]^(y))"(or)"` `E_("cell")=E_("cell")^(@)-(2.303RT)/(nF)LOG""([C]^(l)[D]^(m))/([A]^(x)[B]^(y))` |
|
| 22931. |
What mass of isobutylene is obtained from 37g of tertiary butyl alcohol by heating with20% H_2SO_4 at 363K if the yield is 65%: |
|
Answer» 16g |
|
| 22932. |
Which of the alkane is synthesised from single alkyl halide ? |
|
Answer»
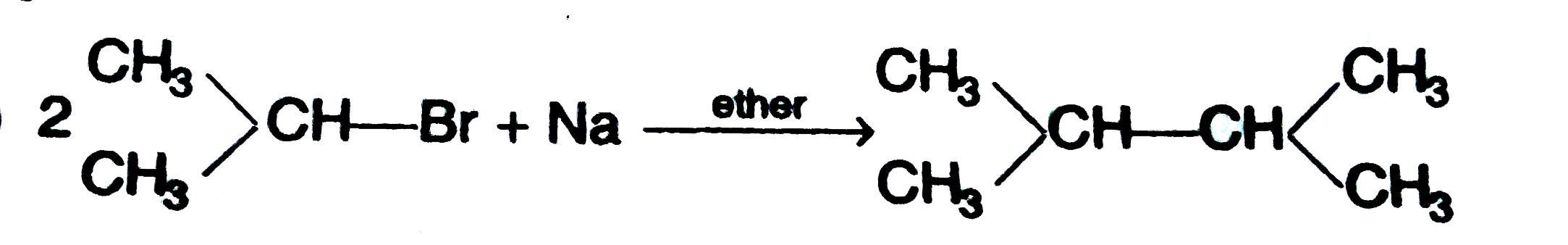 This ALKANE is SYNTHESISED from SINGLE ALKYLHALIDE. |
|
| 22933. |
Which one of the following is correct |
|
Answer» A MINERAL connot be an ore |
|
| 22934. |
When it is used as a reducing agent, the equivalent weight of KHC_(2)O_(4).H_(2)C_(2)O_(4).2H_(2)O is |
|
Answer» the same as its MOLECULAR WEIGHT. |
|
| 22935. |
When a standard solution of NaOH is left in air for a few hours: |
|
Answer» A PRECIPITATE will form |
|
| 22936. |
Tyndall effect is observed in |
|
Answer» TRUE solution |
|
| 22937. |
Which are true for elastomers: |
|
Answer» These are synthetic POLYMERS |
|
| 22938. |
Which of the following is a three dimensional silicate |
|
Answer» MICA |
|
| 22939. |
Which of the following represents largest number of possible isomers? |
|
Answer» `[Ru(NH_3)_4 Cl_2]^+` |
|
| 22940. |
The statement that is not correct for periodic classification of elements is: |
|
Answer» 1. The properties of elements are periodic functions of their atomic numbers `Li lt Be, Be gt B, B lt C, C lt N, N gtO, O lt F` |
|
| 22941. |
When ammonia, is added to green aqueous solution of nickel (II) sulphate, the colour of the solution changes to blue violet. This is caused by: |
|
Answer» |
|
| 22942. |
Zn(s) + Cu^(2+)(aq) rarr Zn^(2+)(aq) + Cu(s) The cell representation for the above redox reaction is |
|
Answer» `CU(s)| Cu^(2+)(aq) || Zn^(2+)(aq) | Zn(s)` |
|
| 22943. |
Which of the following monosaccharides is a pentose |
| Answer» Answer :C | |
| 22944. |
Which of the follwing represents the decreasing order of K_(a) values ? |
|
Answer» `(ii)gt(i)gt(iii)gt(IV)` After releasing `H^(+)` from (ii) group , it forms a more STABLE RESONATING structure of carboxylate ion . In case of (i), the anion formed after release of `H^(+)` is more stable due to presence of `e^(-)` withdrawing `NO_(2)` in benzene ring . In(iii), phenoxide ion formed after release of `H^(+)` is more stable in comparison to acetylide ion of group (iv) which is formed after releasing `H^(+)` fromfrom (iv)so ACIDIC ORDER or `K_(a)` is `(ii)gt(i)gt(iii)gt(iv)` |
|
| 22945. |
Which of the following are the characteristics of chemisorptin ? 1. High heat of adsorption 2. Irreversibility 3. Low activation energy Select the correct answer using the code given below : |
|
Answer» 1 and 2 only |
|
| 22946. |
Which of the following elementsis not found in amino acids ? |
|
Answer» OXYGEN |
|
| 22947. |
Thermodynamicis concerned with: |
|
Answer» Total ENERGY of a system |
|
| 22948. |
Which of the following is reversible sol ? |
|
Answer» Fog |
|
| 22949. |
Which alkyl halide from the following pairs would you expect to react more rapidly by S_(N^(2)) mechanism? . |
|
Answer» Solution :If the leaving group is the same in different isomers of a particular molecular formula, the reactivity of the isomers towards `S_(N^(2))` mechanism decrases with the increase in steric hindrance. In the light of above, the reactivity order in different cases is: (i). `CH_(3)CH_(2)CH_(2)CH_(2)Br` is a primary alkyl HALIDE `(1^(@))`. it is more reactive than the other isomer which is a secondary `(2^(@))` alkyl halide becausae less steric hindrance is caused by primary alkyl group as COMPARED to secondary alkyl group. (ii). `CH_(3)CH_(2)underset(Br)underset(|)(C)HCH_(3)` is a secondary alkyl halide `(2^(@))`. it is more reactive than the other isomer which is a tertiary alkyl halide `(3^(@))` the explanation is the same. (III). Here both the isomers are primary alkyl halides `(1^(@))`. However, the isomer with `CH_(3)` group at `C_(2)` atom exerts more steric hindrance to the attacking nucleophile at `C_(1)` atom as compared TOT he other isomer in which a `CH_(3)` group is attached to `C_(3)` atom. it is , therefore, less reactive.  . .
|
|
| 22950. |
Which is used for sterilization of water in water supply system of cities |
|
Answer» Chlorine |
|