InterviewSolution
This section includes InterviewSolutions, each offering curated multiple-choice questions to sharpen your knowledge and support exam preparation. Choose a topic below to get started.
| 4251. |
Which of the following are examples of metamerism? |
|
Answer» ETHOXYETHANE and 1-methoxypropane |
|
| 4252. |
Which one of the following compounds is not planar? |
|
Answer» `CH_2 = C = CH_2` |
|
| 4253. |
Which of the following is a weakest base : |
|
Answer» Ammonia |
|
| 4254. |
The standard electrode potential are for the following reactions : Fe_((s)) rarr Fe_((aq.))^(2+) + 2e , E^(@) = 0.48 V Cr^(3+) + e rarr Cr_((aq.))^(2+) , E^(@) = -0.41 V If excess of Fe_(s) is added to a solution in which [Cr^(3+)] = 1M, what will be maximum value of [Fe^(2+)] when equilibrium is attained at 298 K ? |
|
Answer» |
|
| 4256. |
The time for half change in a first order decomposition of a substance A is 60 seconds. Calculate the rate constant . How much of A will be left after 180 seconds? |
|
Answer» Solution :(i) Order of reaction =`1, t_(1//2)= 60, "seconds" , k = ? ` We know that , `k=(2.0303)/(t_(1//2))` `k = (2.303)/60 = 0.01155s^(-1)` (II) `[A_0]=100% , t = 180 s, k = 0.01155 " seconds "^(-1) , [A] = ? ` For the FIRST order reaction `k=(2.303)/t log.([A]_0)/([A])` `0.01155=(2.303)/180log.(100/([A]))IMPLIES(0.1155xx180)/(2.303)=log(100/([A]))` 0.9207=log100-log[A] log [A] = log 100 - 0.9207 log [A] = 2 - 0.9207 log [A] =1.0973 [A] = antilog of (1.0973) [A] = 12.5% |
|
| 4257. |
What is Deltan_(g) for the combusion of 1 mole of benzene, when both reactants and products are gases at 298K |
|
Answer» 0 `Deltan=6+3-1-(15)/(2)=+0.5` |
|
| 4258. |
Which of the following will give a primary amine on reduction with LiAIH_(4) ? |
|
Answer» Oxime |
|
| 4259. |
Tritum decays by |
|
Answer» `beta` EMISSION |
|
| 4260. |
Write the structure of the major organic product expected from each of the following reactions: CH_(3)CH_(2)CHCl_(2)underset("alkali")overset("Boil")to |
| Answer» Solution :`CH_(3)CH_(2)CHCl_(2)underset(-2 "NACL ALKALI")OVERSET(BOIL)to (##VMC_NEET_XII_CHE_MOD_05_C20_SLV_026_S01.png" width="80%"> | |
| 4261. |
Which of the following does not contain PX_(4)^(+)type cation in solid phase ? (X=halogen atom) |
| Answer» Answer :A | |
| 4262. |
Which one of the following vitamins deficiency causes rickets: |
|
Answer» VITAMIN A |
|
| 4263. |
The total number of acyclic isomers including the steroisomers (geometrical and optical), with the molecular formula C_(4)H_(7)Cl is:- |
|
Answer» 12 |
|
| 4264. |
Which one of the following complexes will have four isomers? |
|
Answer» `[Co(EN)_(3)]Cl` (i) GEOMETRICAL isomers 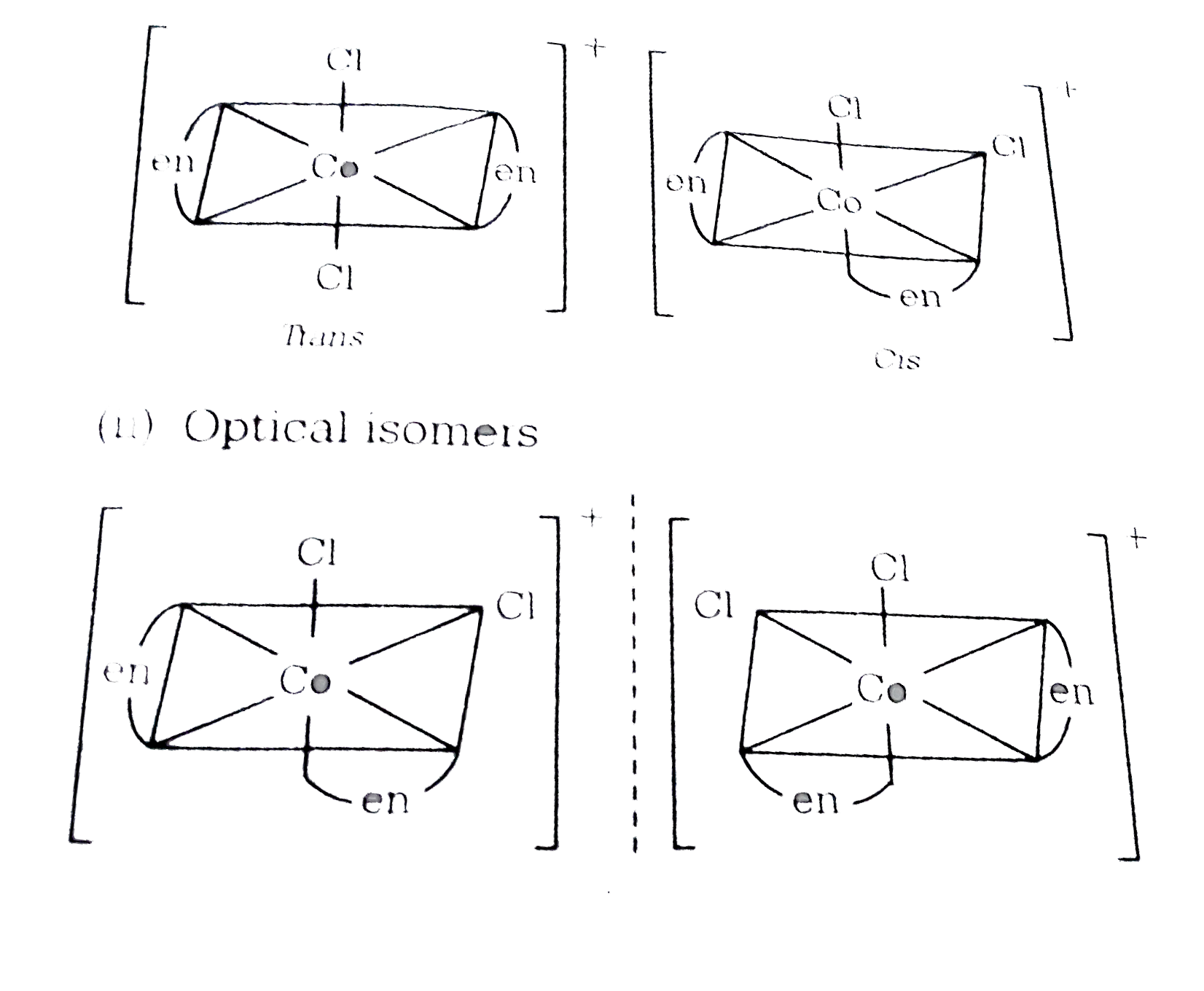
|
|
| 4265. |
Whenlimestone isheated,CO_ 2is givenoff.Themetallurgical operationis |
|
Answer» SMELTING |
|
| 4266. |
Which type of hybridisation is possible in [Ni(CN)_(4)]^(2-) and [Pt(NH_(3))_(4)]^(2+) ? |
|
Answer» `dsp^(2)` |
|
| 4267. |
Van Arkel method can be applied for the purification of: |
|
Answer» B |
|
| 4268. |
Which one is Isoprene ? |
|
Answer» `CH_(2) = CH_(2)` |
|
| 4269. |
what is the shortest wavelength line in Paschen series of Li^(2+)ion (R is Rydberg constant) |
| Answer» Answer :A | |
| 4270. |
Which of the reactions represented in these diagram will show the greatest increase in rate for a given increase in temperature ? |
|
Answer» REACTION I foraward |
|
| 4271. |
What is natural rubber? |
|
Answer» cis-1, 4-polyisoprene |
|
| 4272. |
Which substance is added in chloroform before the use of it as anesthetic ? |
|
Answer» Methyl ethyl ketone |
|
| 4273. |
What are the units of the rate constant of a reaction in which the half life is doubled by halving the initial concentration of reactants. |
|
Answer» `M-s^(-1)` |
|
| 4274. |
Which of the folowing set of molecules have same shape but different hybridisation ? |
|
Answer» `XeO_(4), SF_(4)` |
|
| 4275. |
When CH_(3)COOH reacts with CH_(3)-Mg-X |
|
Answer» `CH_(3)COX` is formed |
|
| 4276. |
The source from where most of helium is obtained at present is |
|
Answer» SUN |
|
| 4277. |
What chemical principle isinvolvedin choosing a reducingagent for gettingthe metalfrom itsoxideore ? Considerthe metaloxides Al_2O_3 andFe_2O_3 , andjustifythe choiceof thereducingagent. |
|
Answer» Solution :Thechemicalprincipleinvolvedin thereducing of metaloxidestometalsis THATAT any giventemperature,any metal will reducetheoxideof othermetalswhich lie aboveit intheEllinghamdiagram. This isbecuasethe standard free ENERGY CHANGE (`Delta_rG^(@)` ) ofthecombinedredox reactionwill be- ve by an amountequal tothe difference in` Delta_f G^(@) `ofthetwometaloxides atthat temperature. It isevident that the`Delta_fG^(@)`curvefor theformationFeO `(or Fe_2O_3` not shownin thediagram )lies above the ` Delta _fG^(@)`curveforthe formation of`Al_2O_3`. Therefore, Al canreduce` FeOor Fe_ 2 O_ 3 ` to `2Al+2FeOoverset ( Delta ) to Al_2O_3+3Fe or "" 2 Al+ Fe_2O_3 overset (Delta ) to Al_2O_3 +2 Fe` Febut thereverseis not TRUE, i.e., Fecannotreduce `Al_2 O_3 `to` Al` ` ""2 Fe+Al_2 O_3 toFe_2O_3 +2Al ` Above 1073 K, theC, CO curvelies below Fe, FeO curve,therefore, above 1073, C can reducesFeO toFe. ` FeO+C overset ( gt 1073K) toFe+CO` In contrast, below 1073 K, the CO, `CO_2 `cuve lies below Fe, FeO curve, therefore,below 1073 K, CO canreduce FeOto Fe. ` "" FeO+COoverset ( lt 1073 K ) toFe+ CO _ 2` |
|
| 4278. |
What is activated charcoal ? |
| Answer» SOLUTION :It is the CHARCOAL TREATED with superheated STEAM to increase its ABSORPTION power. | |
| 4279. |
Which of the following methods of expressing concentration are independent of temperatuer? |
|
Answer» Molarity |
|
| 4280. |
Which of the following is CORRECT expression of calculating the cell potential of an electrochemical cell ? |
|
Answer» `E=E^(@)-(RT)/(nF)In([PRO"DUCTS])/([REACTANTS])` |
|
| 4281. |
Which one of the following is a peptide hormone ? |
|
Answer» Adrenaline |
|
| 4282. |
Treatment of benzene with CO//HCl in the presence of anhydrous AlCl_(3)//CuCl followed by reaction with Ac_(2)O//NaOAc gives a compound X. Reaction of X with H_(2)//Pd//c followed by treatment with H_(3)PO_(4) gives another compound Y. The compound Y is : |
|
Answer»
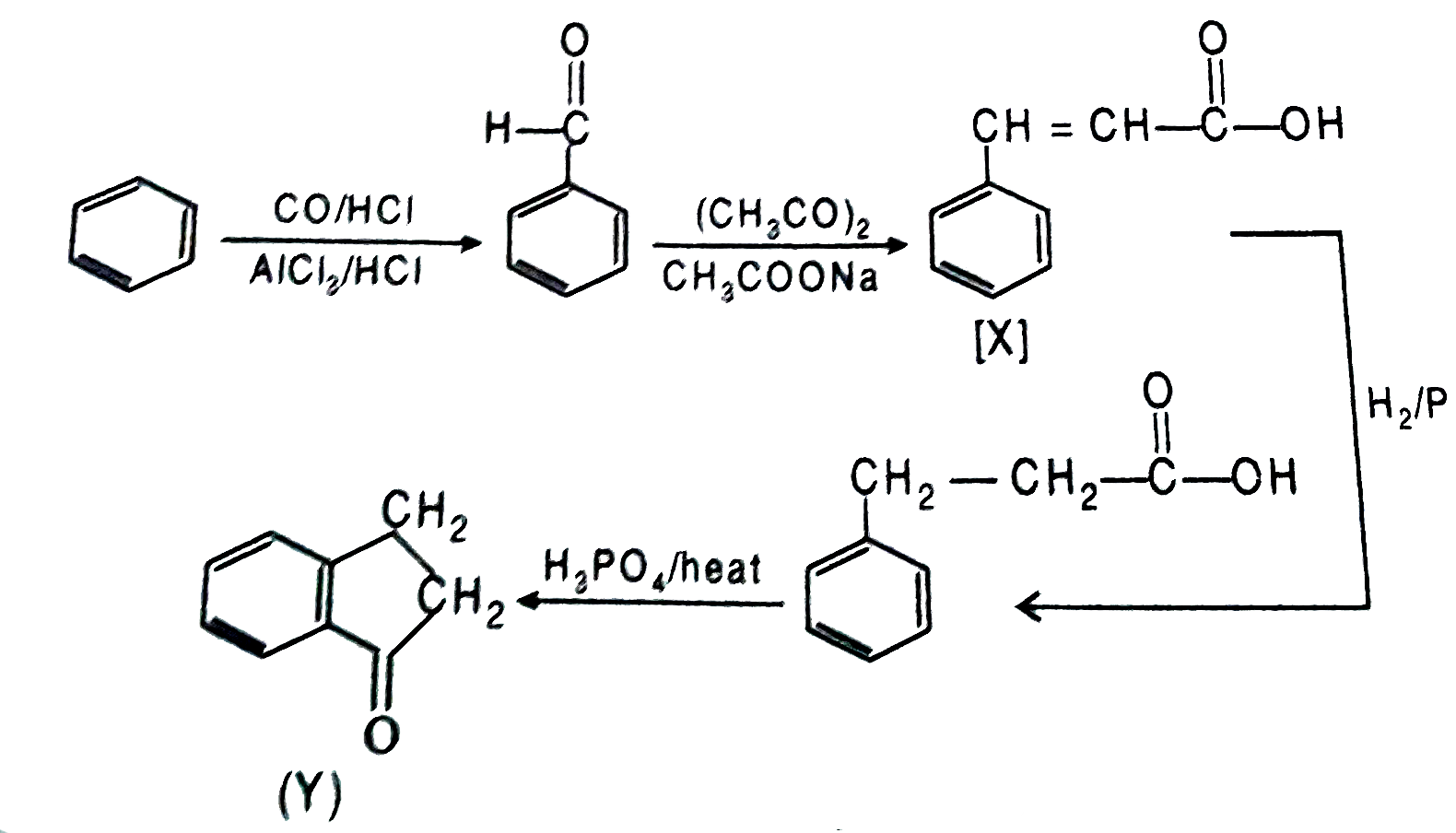
|
|
| 4283. |
The solubility of N_(2) in water at 300K at 300K and 500 torr partial pressure 0.01 gL^(-1). The solubility (in g L^(-1) ) at 750 torr partial pressure is : |
|
Answer» 0.0075 `(p_(1))/(p_(2))=(s_(1))/(s_(2))implies500/750=0.01/(s_(2))impliess_(2)=0.015gL^(-1)` |
|
| 4284. |
What is the maximum possible van't hoff factor of COC13.. xNH_(3) (assume all ammonia is coordinated) |
|
Answer» |
|
| 4285. |
Which of the following is purified by sublimation if the impurities are non-volatile ? |
|
Answer» CANE sugar |
|
| 4286. |
Why are carboxylic acids called fatty acids? |
| Answer» SOLUTION :Since the higher acids such as palmitic acid, STEARIC acid, oleic acid, etc., were first OBTAINED by SAPONIFICATION of OILS and fats. | |
| 4287. |
The vapour pressure of a solvent is 60 torr while that of its dilute solution is 52 torr. The mole fraction of the solvent is : |
|
Answer» <P>0.867 `(p^(@)-p_(s))/(p^(@))=X`(solute) `(60-52)/(60)=x` (solute) or `x` (solute)`=0.133` `x`(solvent)`=1-0.133=0.867` |
|
| 4288. |
Which of the following structures does not contain any chiral C atom but represent the chirality in the structure ? |
|
Answer» 2-Ethyl-3-hexene |
|
| 4289. |
Which of the following is the major product formed when phenol is treated with dilute nitric acid at 293 K? |
|
Answer» m-Nitrophenol |
|
| 4290. |
Which of the following represents the correct order of increasing first ionisation enthalpy for Ca, Ba, S, Se and Ar ? |
|
Answer» CA S Ba Se Ar In a period I.E in general INCREASES from left to RIGHT and therefore. `ArgtS`and`SegtCa` Hence correct order is `BaltCaltSeltSltAr` |
|
| 4291. |
Two jars A and B are filled with hydrocarbons. Br_2 in C CI_4 is added to these jars. A does not decolourises whereas B decolourlises. What are A and B: |
|
Answer» ALKANE and alkene |
|
| 4292. |
Which branched chain isomer of the hydrocarbon with molecular mass 72mu gives only one isomer of mono substituted alky halide ? |
|
Answer» Tertiary BUTYL chloride |
|
| 4293. |
What happens when ethyl iodide is heated with alcoholic KOH. |
|
Answer» Solution :When ethyl BROMIDE is TREATED with alcoholic KOH solution ETHYLENE is formed. ` underset(ethylbromide)(C_2H_5Br)+KOH_(ALC.) rarr KBR +H_2O+underset(ETHYLENE)(C_2H_4)` |
|
| 4294. |
Which of the following regulates the metabolism of sugars |
|
Answer» Thyroid |
|
| 4295. |
Which by - product is obtained in the manufacture of phenol from cumene ? |
|
Answer» Acetone |
|
| 4296. |
Which polymer is used to prepare fishing net ? |
| Answer» Solution :Nylon-6,6 | |
| 4297. |
Write down the reaction are involved in the extraction of lead. PbS+2O_(2)rarr A,, 2PbS+3O_(2)rarr B +2SO_(2): PbS+2PbO rarr C + 2SO_(2) .A,B,Crespectively |
|
Answer» PbS`,PbSO_(4)` PBO |
|
| 4298. |
The specific conductance of 0.1N KCl solution at M 23^@C is 0.012 ohm^(-1)cm^(-1). The resistance of cell containing the solution at the same temperature was found to be 55 ohm. The cell constant will be |
|
Answer» `0.142 CM^(-1)` |
|
| 4299. |
Which of the following structures represents thymine ? |
|
Answer»

|
|
| 4300. |
Which of the following change the colour of the aqueous solution of FeCl_(3) |
|
Answer» `K_(4)[Fe(CN)_(6)]` `2FeCl_(3)+3H_(2)S to Fe_(2)S_(3)+6HCL` `3NH_(3)CNS+FeCl_(3)to underset(("blood RED"))(Fe(CNS)_(3))+3NH_(4)Cl` `FeCl_(3)+3KCNSto underset(("Blood red"))(Fe(CNS)_(3))+3KCl` |
|