Saved Bookmarks
| 1. |
a. A sample of 0.50g of an organic compound wastreated according to Kjeldahal's method. The ammonia evolved was aboserved in 50 ml of 0.5M H_(2)SO_(4). The residualacid required 60 mL of 0.5M solution of NaOH for neutralisation. Find the percentage composition of hnitrogen in the compound. b. On analysis, 0.2g of a monobasic acid gave 0.505 gm of CO_(2) and 0.0864gm H_(2)O. 0.305 gm of this acid required25 ml of M//10 NaOH solution for neutralisaton. Find the molecular formula of the acid. c. A liquid aromatic organcvi compound (A) conatining carbon (92.3%) and hydrogen (7.7%)decoluidsed KMnO_(4) and on ozonolysis gave methanal and another compound (B). The molecular mass of (A)is 104. On treatmentwith a suitablecatalyisis, (A)gave a high molecularmass solid product (C)havingthe same empirical formula as that of compound(A). Compound (C) is used in making toys and household goods. Identify (A),(B), and (C) and explain the reactions. d. A sample of 0.246 gm of an organic compound gave 0.198 gm fo CO_(2) and 0.104 gm of H_(2)O on completecombusion. .37gm of the compoundgave 0.638 gm of silver bromide in Carius method. What is the molecular formula of the compound if its molecular mass is 109. |
|
Answer» Solution :a. TOTAL acid `= 50xx0.5xx2 = 50 mEq`. Excess acid `= 60xx0.5 = 30 mEq`. Acid used to neutralise `NH_(3) = 50 - 30 = 20 mEq`. Perventage of `N = (1.44xx mEq. "of acid")/("Wt fo compd".)` `= (1.4xx20)/(0.5) = 56%` b. Caculate of emprical formula. MASS of sample `= 0.20gm`, mass of `CO_(2)` formed `= 0.505gm` mass of water formed `= 0.864 gm`. Percentage of carbon `= (12xx0.505xx100)/(44xx0.20) = 69%` Percentage of hydrogen `= (2xx0.086xx100)/(18xx0.20) = 4.8%` Percentage of oxygen `= 100-(69+4.8) = 26.20%` Emprical formula `= C_(7)H_(6)O_(2)` 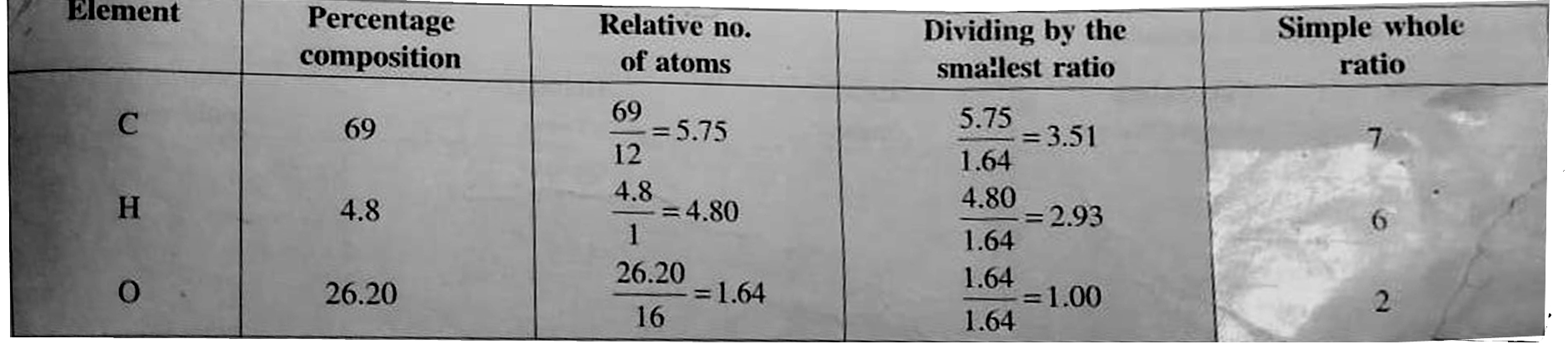 `40 gm` of `NaOH` would meutralise `1 mol` of amononbasic acid `NaOH = 25 xx (1)/(10) = 2.5 mEq. = 2.5xx10^(-3)xx40 = 0.1 gm` `0.1gm` of `NaOH` neuralies `0.305 gm` acid `40gm NaOH` neutralies `(0.305xx40)/(0.1)= 122gm` Molecular mass of acid `= 122 gm mol^(-1)` `n = ("Mol. formula mass")/("EMPIRICAL formula mass") = (122)/(122) = 1` Molecular formula of acid `= (C_(7) H_(6) O_(2))_)1) = C_(7) H_(6) O_(2)` c. 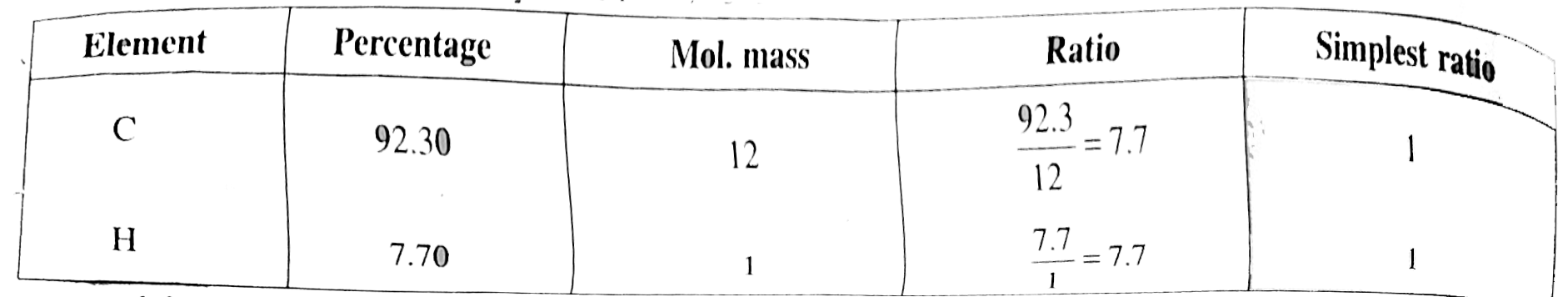 Empirical formula `= CH` Mol mass of `(A) = 2xxV.D. = 2xx52 = 104` `n = (104)/(13) = 8` Molecule formula `= C_(8) H_(8)` Decree of unsaturation `= [(2xx8+2) - 8]//2 = 5^(@)` `5^(@) unsaturation shows that it is can aromatic COMPOUND, `4^(@)` due to BENZENE ring. 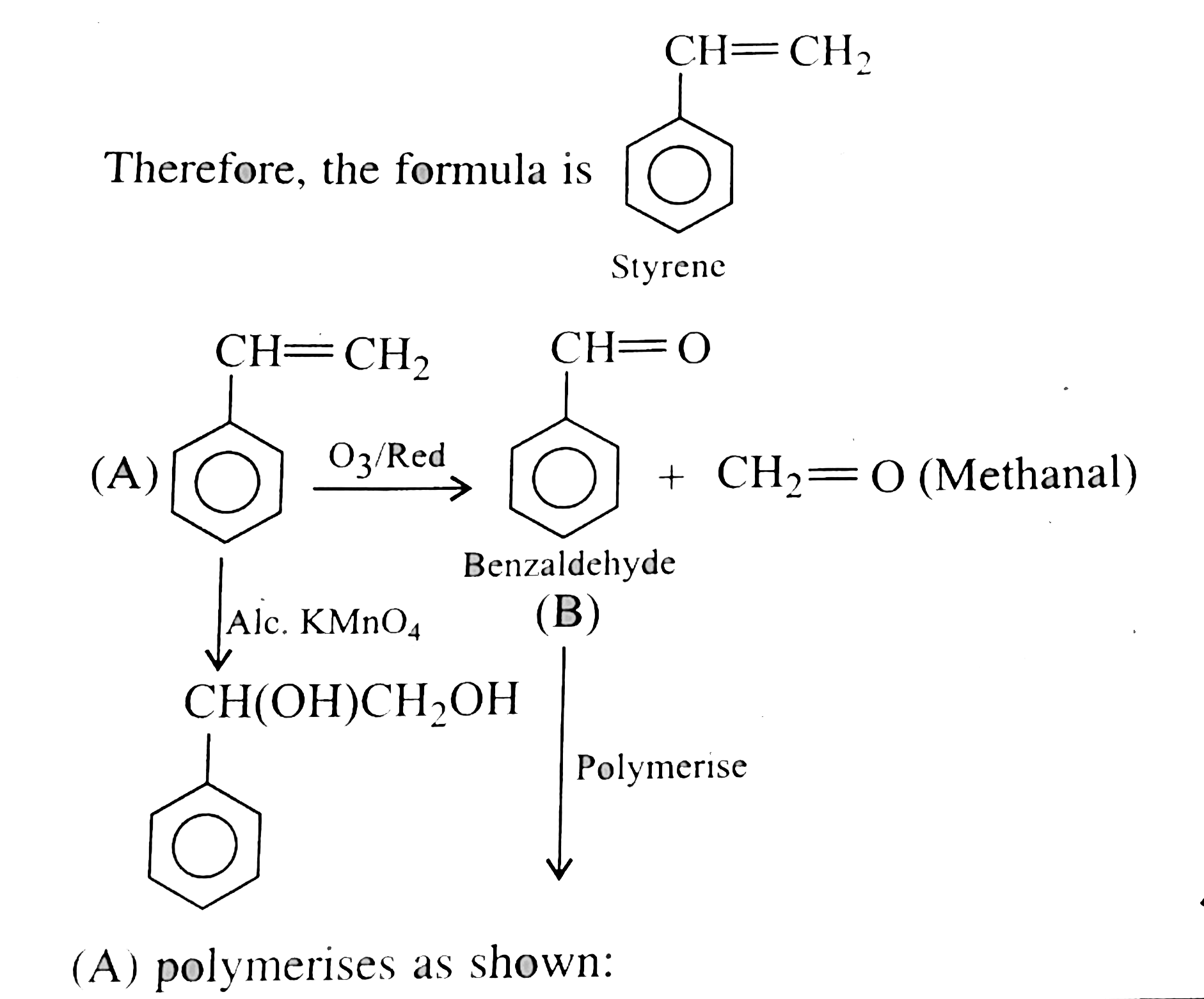 `(A)` plymerises as shwon: `n(overset(Ph)overset(|)(CH)= CH_(2)) rarr - overset(Ph)overset(|)(CH) - CH_(2) - overset(Ph)overset(|)(CH) - CH_(2) -` 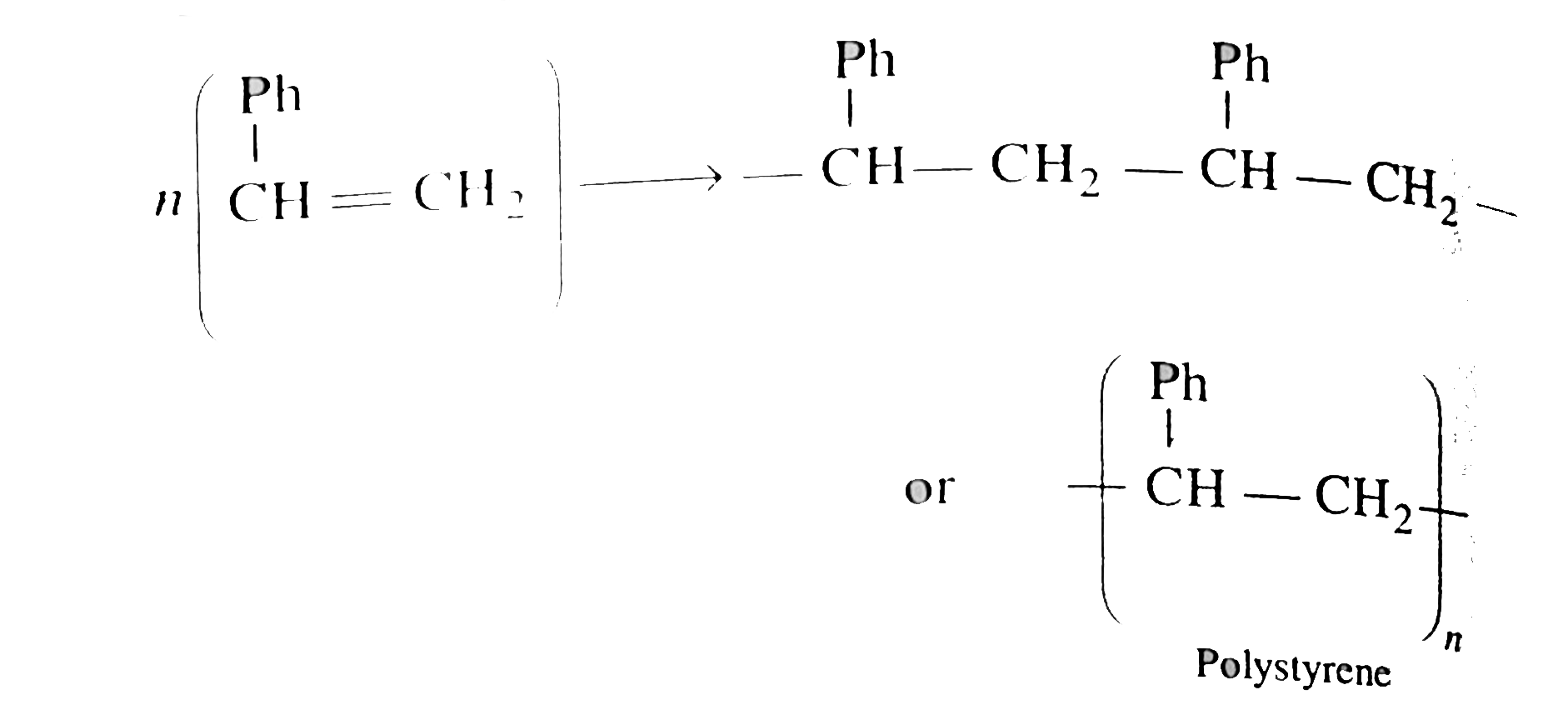 d. Mass of organic compound`= 0.26 gm` Mass of `CO_(2) = 0.198 gm` Mass of `H_(2)O = 0.1014 gm` Percentage of `C = (12xx0.198xx100)/(44xx0.246) = 21.95%` Percentage of `H = (2xx0.1014xx100)/(18xx0.246) = 4.58%` Percentage of bromic `= (80xx0.638xx100)/(188xx037)` `= 73.37%`  Emprical formula `= C_(2) H_(5) Br`, Molecular mass `= 109` `n = 109//109 = 1` Molecular formula `= (C_(2) H_(5)Br)_(1) = C_(2)H_(5)Br` |
|
Discussion
No Comment Found
Related InterviewSolutions
- Which of the following compounds is not cleaved by HI even at 525 K ?
- To a 25 mL H_(2)O_(2) solution excess of an acidified solution of potassium iodide was added. The iodine liberated required 20 " mL of " 0.3 N sodium thiosulphate solution Calculate the volume strength of H_(2)O_(2) solution.
- The suggested mechanism of a reaction is : (a) A+BhArrD("fast) "(b)A+Drarr2C("slow")Write the balanced equation of the reaction if its experimentally deduced rate equation is , rate k=[A]^(2)[B] Find the intermediate formed during the course of the reaction . Does the predicted rate law from the mechanism match the experimental rate law ?
- Which of these changes with time for a first-order reaction A Rate of reaction B . Rate constant C . Half-life
- What is the hybridisation of central atom in the product obtained along with hydrofluoric acid when complete hydrolysis of Xenon Hexa Fluoride takes place ?
- Which of the following amino acid forms sulphide bond in polypeptide
- Which of following pair is Diastereomers:
- What is the major product of the following reaction CH_3C-=C-CH_2-CH_3overset("1 mole of " Cl_2)to
- Which polymer is used in petrol tank linings ?
- Which of the following carbohydrates are branched polymer of glucose ?