Saved Bookmarks
| 1. |
a. The treatment of RX with aqueous KOH leads to the information of alcohols but in the presence of alcholic KOH or NaOH, alkenes are themajor products. Expalin why. b. CHF_(3) is less acidic than CHCl_(3). Explain. c. Wurtz reactionin case of tert-alkyl halide fails. Explain. d. Dipole moment of C_(6) H_(5) Cl is lower thanthat of C_(6) H_(11) Cl (cuclohexul choride). Expalin why. e. Why should Grignardreagent be preparedunder anthydrous conditions? f. Why does p- dichlorobenzene have higher melting point and lower solubility than o- and m- isomers ? |
|
Answer» Solution :a. `NaOH` or `KOH` is COMPLETELY ionised in aquioes solution to give `Oh` ions, which acts as a strongnuclophile, so `SN` reactiontakes placewith `RX` to give alcholos. Moreover, `OH` ions are hydrarded or solvated in aquires soltion which reduces the basic character of `overset(ᶱ)(O)H` that cannot abstact acidic `beta-H` atom of `EX` to from alkene. In case of alcholic solution of `NaOH` or `KOH, overset(ᶱ)(O)H`reacts with `ROH` to fromRO` (alkoxide ion) which is a stronger base than `overset((-))(O)H`, and `RO^((-))` can abstract acidic `beta-H` atom of `RX` easily to form alkense. d `-I` effect of `FgtCl`. According to `-I` effect, `CHF_(3)~ should be more acidic than `CHCl_(23)`. but this is not observed. the conjugate base `overset(..)overset((-))(C)Cl_(3)` is resonance stabilised due to the presence of `d`-orbital in `Cl[2p(C)-3d(Cl)]` overlap. But conjugate base `overset(..)overset((-))(C)F_(3)` is not resonance stabilised due to the absence of `d`-orbital in `F`. 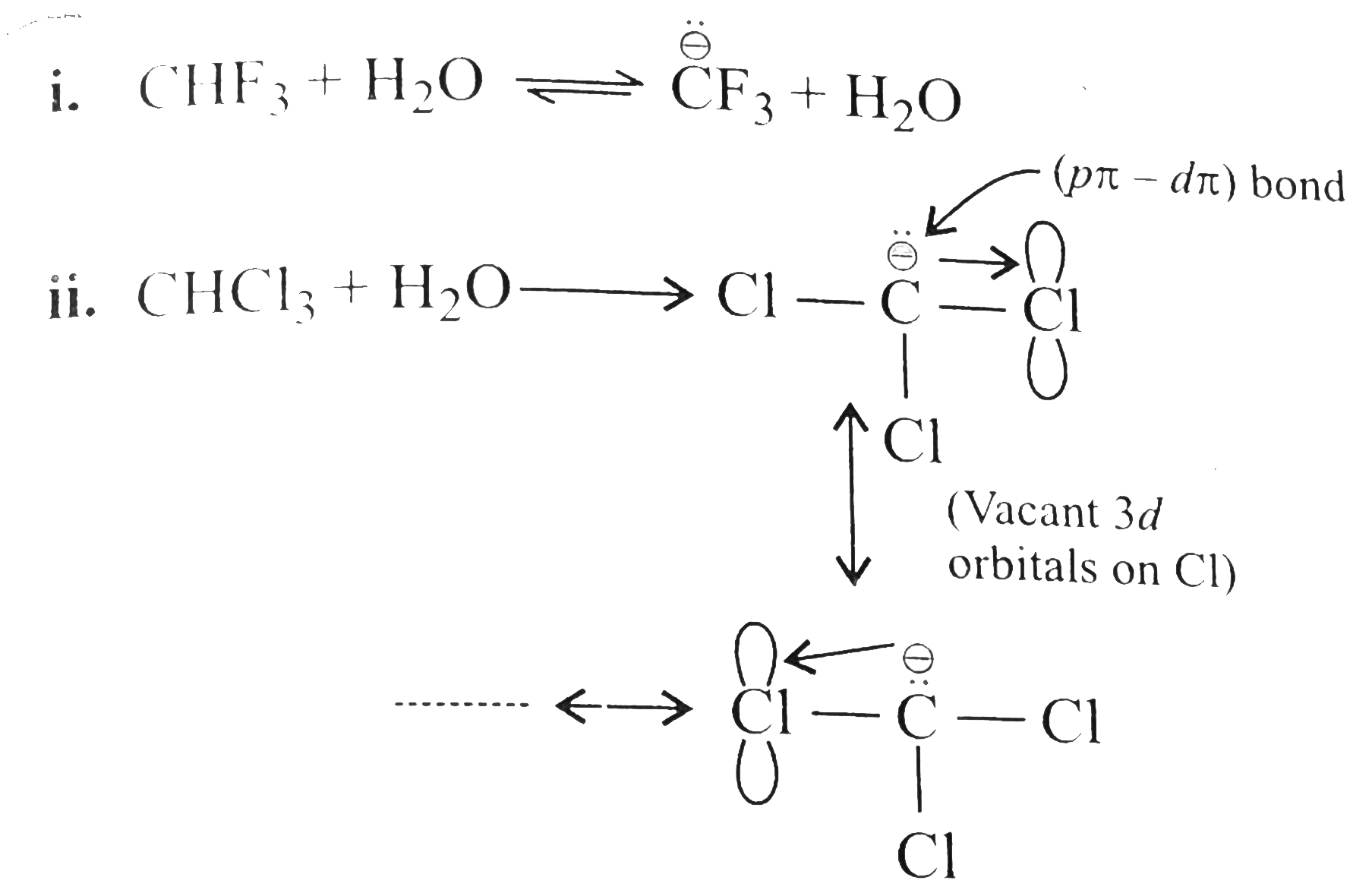 (c) `Me_(3)C-X+2Natounderset("t-Butylsodium")Me_(3)overset((-))(C)-overset(o+)(Na)+NaX` `t`-ALKYL halides undergo dehydrohalogenation in the presence of storng base such as `Na` metal rather thanWurtz reaction. 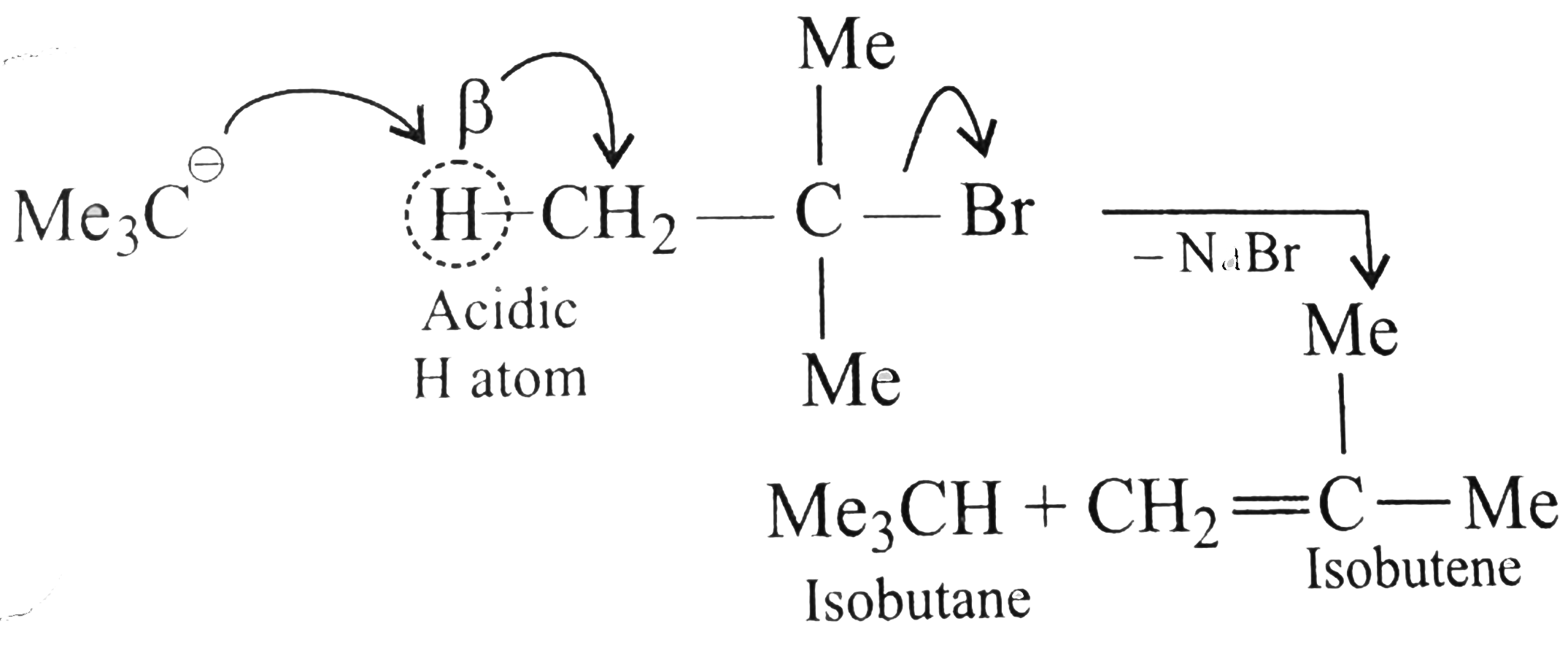 Therefore, `1^(@)` and `2^(@) RX` undergo Wurtz reaction, while `3^(@) RX` undergo dehydroghalogenation to give alkenes. 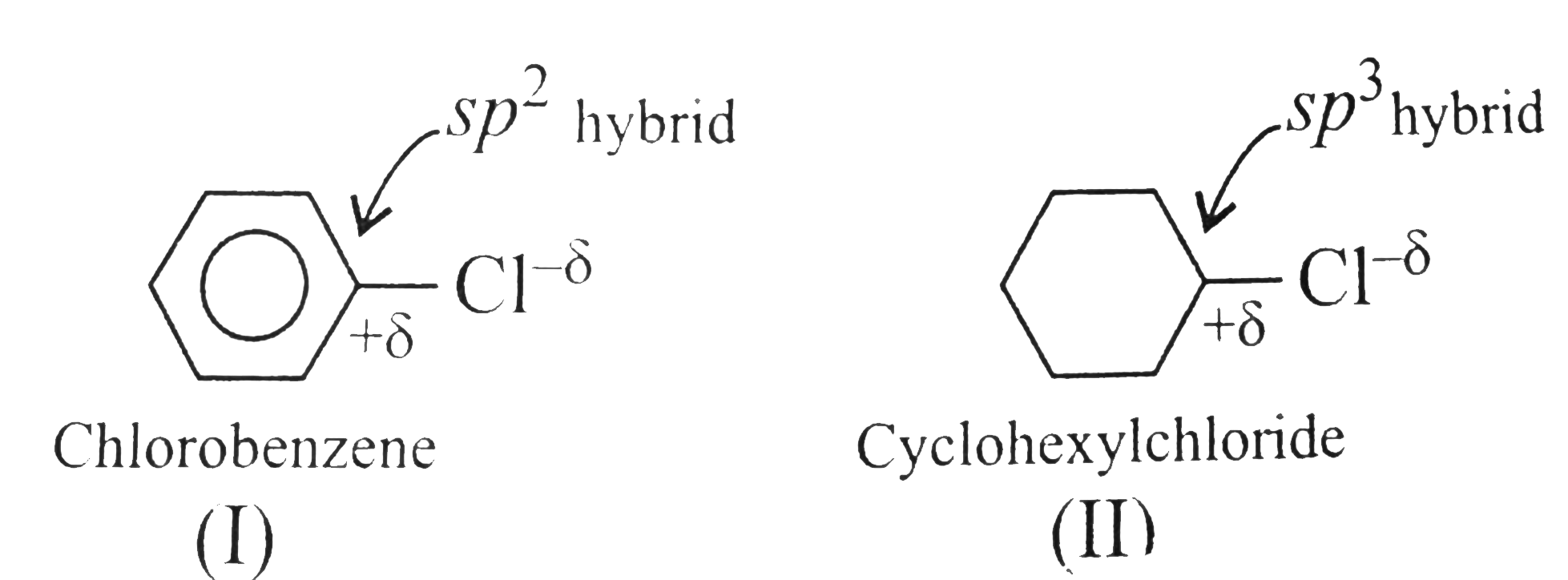 (i) `sp^(2)` -HYBRID `C` atom is more `EN` than `sp^(3)` -hybrid `C` atom . (ii) Therefore `sp^(2)` -hybrid `C` atom of `(C-Cl)` bond in (i) has less tendency to release electrons to `Cl` than ans `sp^(3)` -hybrid `C` of `(II)`. (iii) Therefore,`(C-Cl)` bond in `(I)` less polar than in (II), i.e., magnitude opf `delta^((-))` charge is less on `Cl` atom of `(I)` than that of (II) (iv) Due top resonance in (I), the `(C-Cl)` bond in `(I)` acquire some double bond character and is shorter than that in (II). (v). Since `mu=qxxd` (charge `xx`distance), therefore, `(I)` has lower `mu` than `(II)`. (e) Grignard reagents are very reactive and they react with moisture present INT the reaction mixture, therefore they must be prepared under anhydrous conditions. 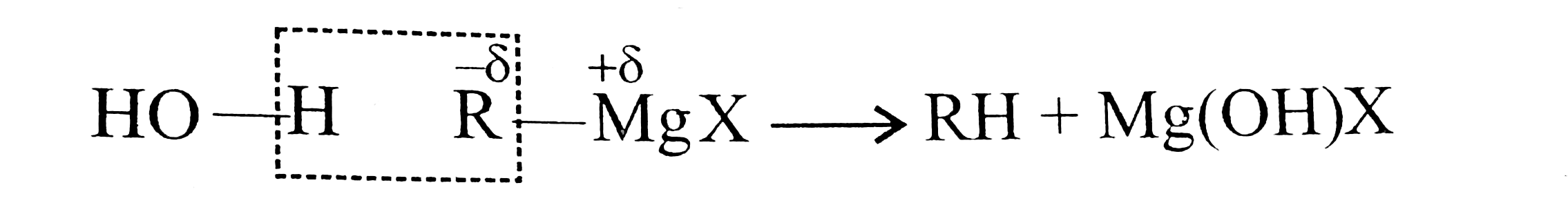 (f) Due to symmetrical packing in its crystal lattice of `p`-isomer, they have stronger intermolecular force of attraction than `o-` and `m-` isomers. Therefore, large amount of energy is required for its melting than for the `o-` and `m-`isomers. The enthalpy of intermolecular force of attraction in `p`-isomer is greater than the enthalpy of dissolution. hence `p-`isomer has lower solubility than its isomers. |
|
Discussion
No Comment Found
Related InterviewSolutions
- Which of the following compounds is not cleaved by HI even at 525 K ?
- To a 25 mL H_(2)O_(2) solution excess of an acidified solution of potassium iodide was added. The iodine liberated required 20 " mL of " 0.3 N sodium thiosulphate solution Calculate the volume strength of H_(2)O_(2) solution.
- The suggested mechanism of a reaction is : (a) A+BhArrD("fast) "(b)A+Drarr2C("slow")Write the balanced equation of the reaction if its experimentally deduced rate equation is , rate k=[A]^(2)[B] Find the intermediate formed during the course of the reaction . Does the predicted rate law from the mechanism match the experimental rate law ?
- Which of these changes with time for a first-order reaction A Rate of reaction B . Rate constant C . Half-life
- What is the hybridisation of central atom in the product obtained along with hydrofluoric acid when complete hydrolysis of Xenon Hexa Fluoride takes place ?
- Which of the following amino acid forms sulphide bond in polypeptide
- Which of following pair is Diastereomers:
- What is the major product of the following reaction CH_3C-=C-CH_2-CH_3overset("1 mole of " Cl_2)to
- Which polymer is used in petrol tank linings ?
- Which of the following carbohydrates are branched polymer of glucose ?