Saved Bookmarks
| 1. |
Account for the following : (i) Ethers have significant dipole moments. (ii) Ethers have lower boiling points than the corresponding isomeric alcohols but approximately equal to a paraffin with similar molecular mass structure. (iii) HI is better reagent than HBr for cleavage of ethers. (iv) Why a non-symmetrical ether is not prepared by heating a mixture of ROH and R'OH in acid ? (v) Why is it possible to prepare tertiary butyl ethyl ether in good yield by heating tertiar butanol and ethanol ? (vi) Ether are soluble in conc. H_(2)SO_94) but ethers separate out from the solution on addition of water. (vii) Why id diethyl ether used as a solvent for (i) BF_(3) and (ii) RMgBr ? (viii) Sometimes explosion occrs during distillation of an ether sample. (ix) Why [(CH_(3))_(3)C]_(2)O cannotbe prepared either by Williamson's reaction or by dehydration of tertiary butyalcohol ? (x) Why ArO-R ethers are clearved with HI togive RI and ArOH rather than ArI and R-OH ? (xi) Which of the following is the correct method for synthesising methyl tert-butyl ether and why ? (a) (CH_(3))_(3)C-Br+NaOMe rarr (b) CH_(3)+NaO -T-Bu rarr (xii) 2,2-Dimethyl oxirane can be cleaved by acid (H^(+)) . Write mechanism. (xiii) Write the equations of the reaction of hydrogen iodide with : (i) 1-Propoxypropane (ii) Methyoxybenzene 9iii) Benzyl ethyl ether |
|
Answer» Solution :(i) Ethers are weakly polar. The C-O-C bond angle is about 383K and the C-O moments do not CANCEL each other . (ii) ROH molecules have strong intermolecular attractive forces because of hydrogen bonding that is absent in ethers having two polarity. Hence, the boiling points of ethers are low. (iii) HI is a stronger acid than HBr and thus, oxonium salt is readily formedwith greater yields. `I^(-)` is also a better nucleophile than `Br^(-)` in `S_(N^(2))` reaction. (iv) A mixture of three ethers, `R-O-R,R'-O-R'` and `R-O-R'` is obtained. (v) When one alcohol is `3^(@)`, the oxonoium ion easily loses water to FORM carbocation, whichis solvated by `2^(@)` or `1^(@)` alcohol to form mixed ether. `Mg_(3)COoverset(+)(H_(2))underset((-H_(2)O))(rarr)Me_(3)overset(+)(C) underset(-H^(+))overset(HOCH_(2)CH_(3))(rarr)Me_(3)C-O-CH_(2)CH_(3)`. (vi) Water is a stronger base than ether and REMOVES the proton from `R_(2)OH^(+)` `R_(2)OH^(+)+H_(2)O rarr R_(2)O +H_(3)^(+)O` (viii) Ethers form peroxide with oxygen. The boiling point of peroxide is higher than that of ether . It is, thus, left as residue in the distillation of ether which is very unstable and decomposes violently on heating . (ix) The product in both the cases is isoubutylene as the tert-butyl carbocation elemination an `H^(+)`. The reaction between `(CH_(3))_(3)overset(+)(C)` on `(CH_(3))_(3)COH` to give an ether is sterically hindered. The instability of di-, tert-butyl ether in sulhuric acid may also be due to steric crowding of `CH_(3)` groups ? 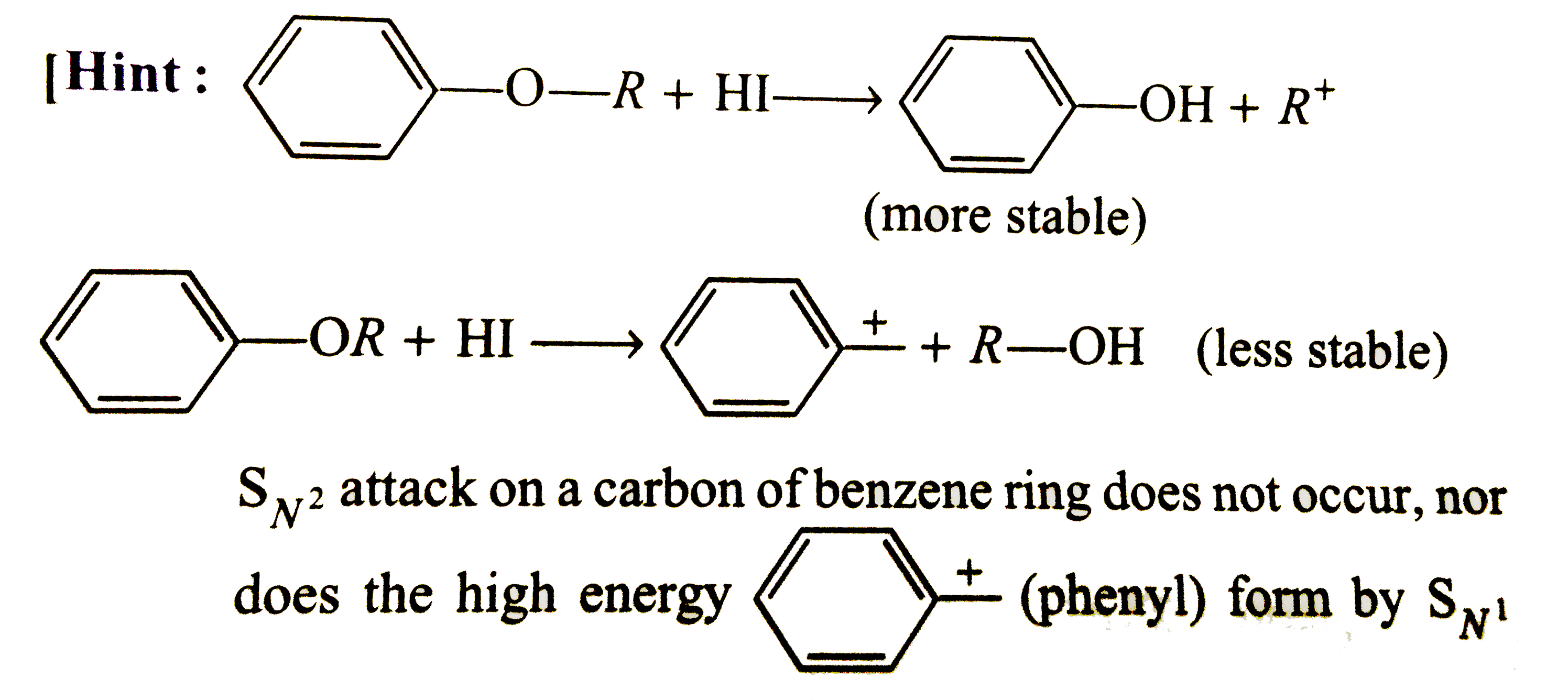 (xi) The ether FORMATION involves nucleophilic SUBSTITUTION of alkoxide ion for halide ion. 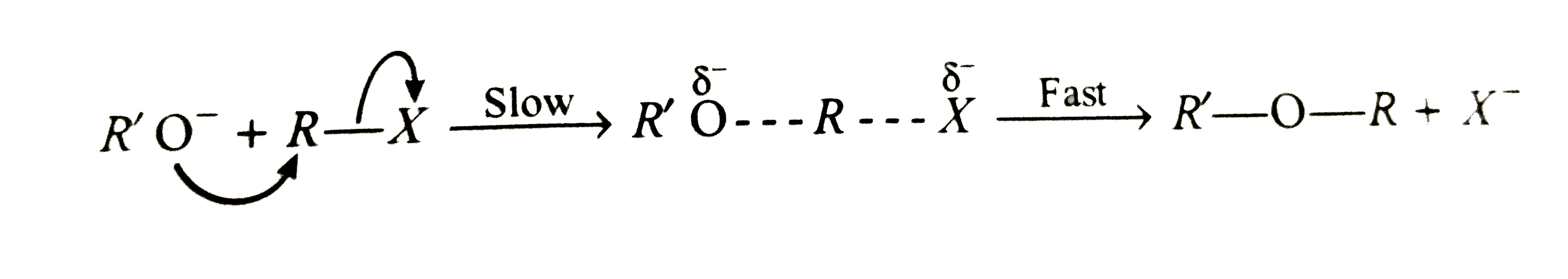 `3^(@)` alkyl halide can also involve elimination of HX to give alkene in the presence of a base. So, it is better to start with `3^(@)` alkoxide and `1^(@)` alkyl halide, i.e., equation (b). (xii) The oxirane ring is cleaved via `S_(N^(2))` mechanism. 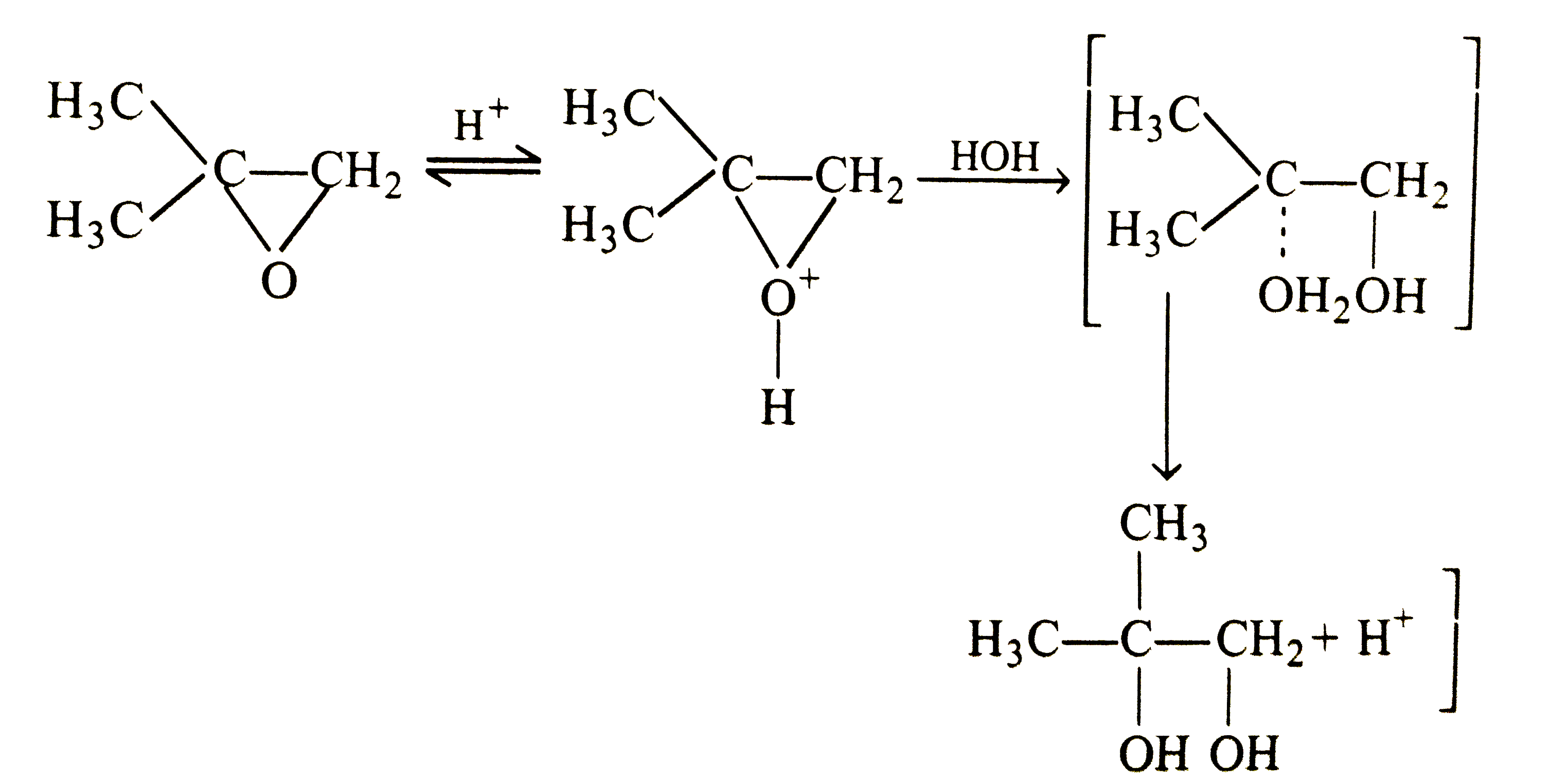 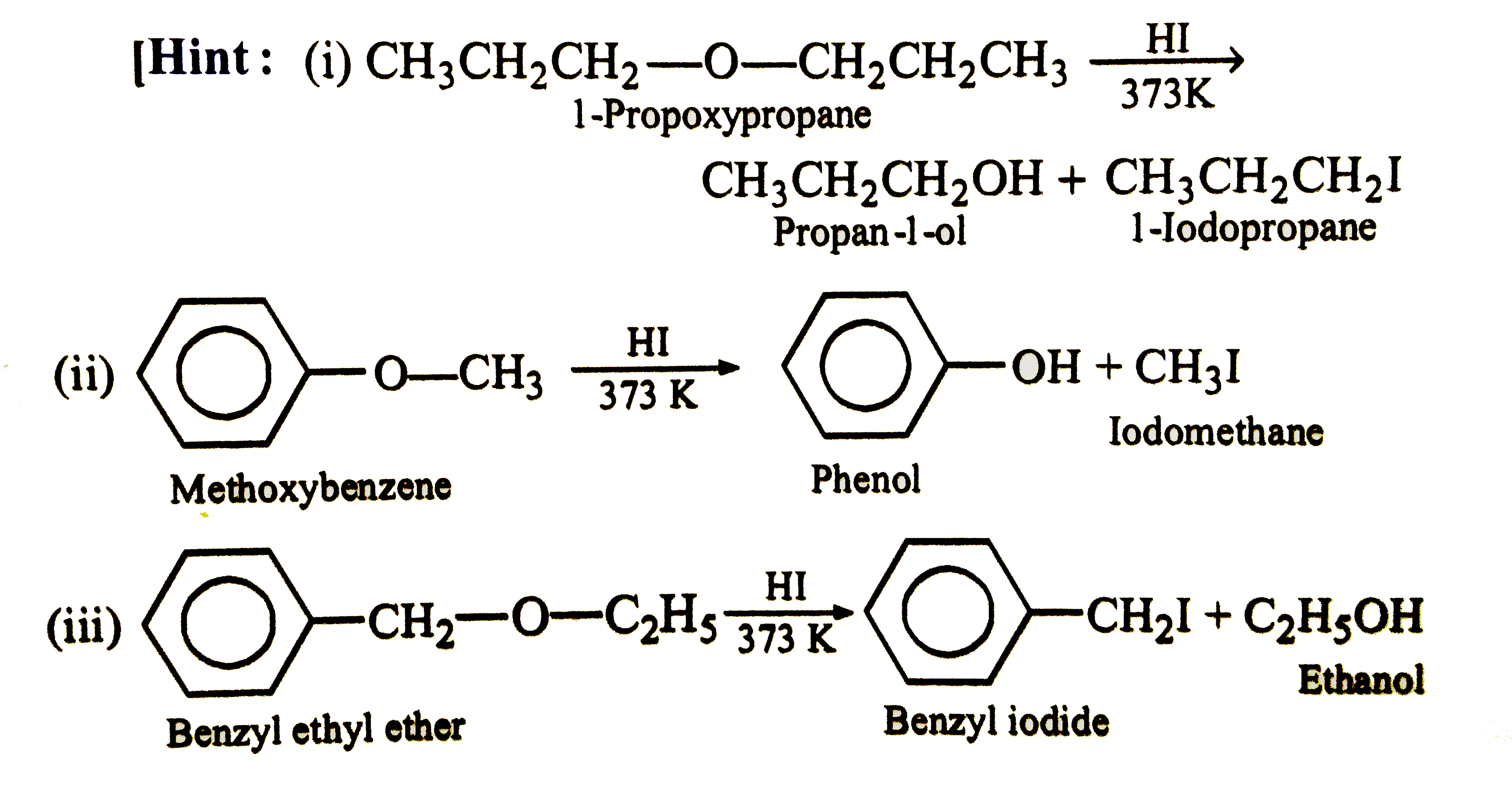
|
|
Discussion
No Comment Found
Related InterviewSolutions
- Which of the following compounds is not cleaved by HI even at 525 K ?
- To a 25 mL H_(2)O_(2) solution excess of an acidified solution of potassium iodide was added. The iodine liberated required 20 " mL of " 0.3 N sodium thiosulphate solution Calculate the volume strength of H_(2)O_(2) solution.
- The suggested mechanism of a reaction is : (a) A+BhArrD("fast) "(b)A+Drarr2C("slow")Write the balanced equation of the reaction if its experimentally deduced rate equation is , rate k=[A]^(2)[B] Find the intermediate formed during the course of the reaction . Does the predicted rate law from the mechanism match the experimental rate law ?
- Which of these changes with time for a first-order reaction A Rate of reaction B . Rate constant C . Half-life
- What is the hybridisation of central atom in the product obtained along with hydrofluoric acid when complete hydrolysis of Xenon Hexa Fluoride takes place ?
- Which of the following amino acid forms sulphide bond in polypeptide
- Which of following pair is Diastereomers:
- What is the major product of the following reaction CH_3C-=C-CH_2-CH_3overset("1 mole of " Cl_2)to
- Which polymer is used in petrol tank linings ?
- Which of the following carbohydrates are branched polymer of glucose ?