Saved Bookmarks
| 1. |
Action of soap is due to emulsification and micelle formation, Comment . |
Answer» Solution :Soap molecule has two parts : Hydrophobic and hydrophilic. The first one attaches with oily DIRT and the second attaches with water. 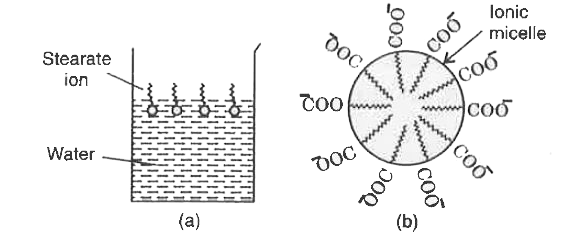 (a) Fig. (a) Arrangement of stearate ions on the surface of water at low concentrations of soap (b) Arrangement of stearate ions inside the bulk of water (ionic micelle) at critical micelle concentrations of soap. As the dirty cloth is agitated in soap solution, several soap molecules arrange themselves radially, each attached with OIL at one end and water on the other end. So, dirt is removed from the cloth and an emulsion of oily dirt and water is formed in the soap solution. Thus, soap HELPS in EMULSIFICATION and washing away of oily dirt from the cloth. 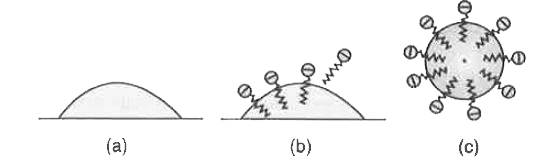 Fig. (a) Grease on cloth (b) Stearate ion arranging around the grease droplet and (c) Grease due. |
|
Discussion
No Comment Found
Related InterviewSolutions
- Which of the following compounds is not cleaved by HI even at 525 K ?
- To a 25 mL H_(2)O_(2) solution excess of an acidified solution of potassium iodide was added. The iodine liberated required 20 " mL of " 0.3 N sodium thiosulphate solution Calculate the volume strength of H_(2)O_(2) solution.
- The suggested mechanism of a reaction is : (a) A+BhArrD("fast) "(b)A+Drarr2C("slow")Write the balanced equation of the reaction if its experimentally deduced rate equation is , rate k=[A]^(2)[B] Find the intermediate formed during the course of the reaction . Does the predicted rate law from the mechanism match the experimental rate law ?
- Which of these changes with time for a first-order reaction A Rate of reaction B . Rate constant C . Half-life
- What is the hybridisation of central atom in the product obtained along with hydrofluoric acid when complete hydrolysis of Xenon Hexa Fluoride takes place ?
- Which of the following amino acid forms sulphide bond in polypeptide
- Which of following pair is Diastereomers:
- What is the major product of the following reaction CH_3C-=C-CH_2-CH_3overset("1 mole of " Cl_2)to
- Which polymer is used in petrol tank linings ?
- Which of the following carbohydrates are branched polymer of glucose ?