Saved Bookmarks
| 1. |
Define : Cryoscopic constant What is the action of hot/concentrated nitric acid on : (a) Arsenic (b) AntimonyDraw the structure of : (a) Orthophosphoric acid (b) Pyrophosphoric acid |
|
Answer» Solution :Cryscopic constant or molal DEPRESSION constant is the depression in frrzing point produced by dissolving 1 mole of solute in 1 kg of solvent . Its units are K kg ` "MOL"^(-1) or .^(@)C "kgmol"^(-1)`or K/molal . (a) When the concentrated nitric acid reacts with the arsenic it forms arsenic acid . ` {:(5 HNO_(3),+,As,rarr,H_(3)AsO_(4),+,5NO_(2),H_(2)O),(("CONC."),,,,("Arsenic acid"),,,):}` (b) When the hot or concentrated nitric acid react with the antimony it forms a COMPOUND named as antimony pentoxide . ` {:(4Sb,+,20HNO_(3),to,Sb_(4)O_(10),+,20NO_(2),+,10H_(2)O),(,,("conc."),,("Antimony oxide"),,,,):}` ( a) Orthophosphoric Acid `(H_(3)PO_(4))` : 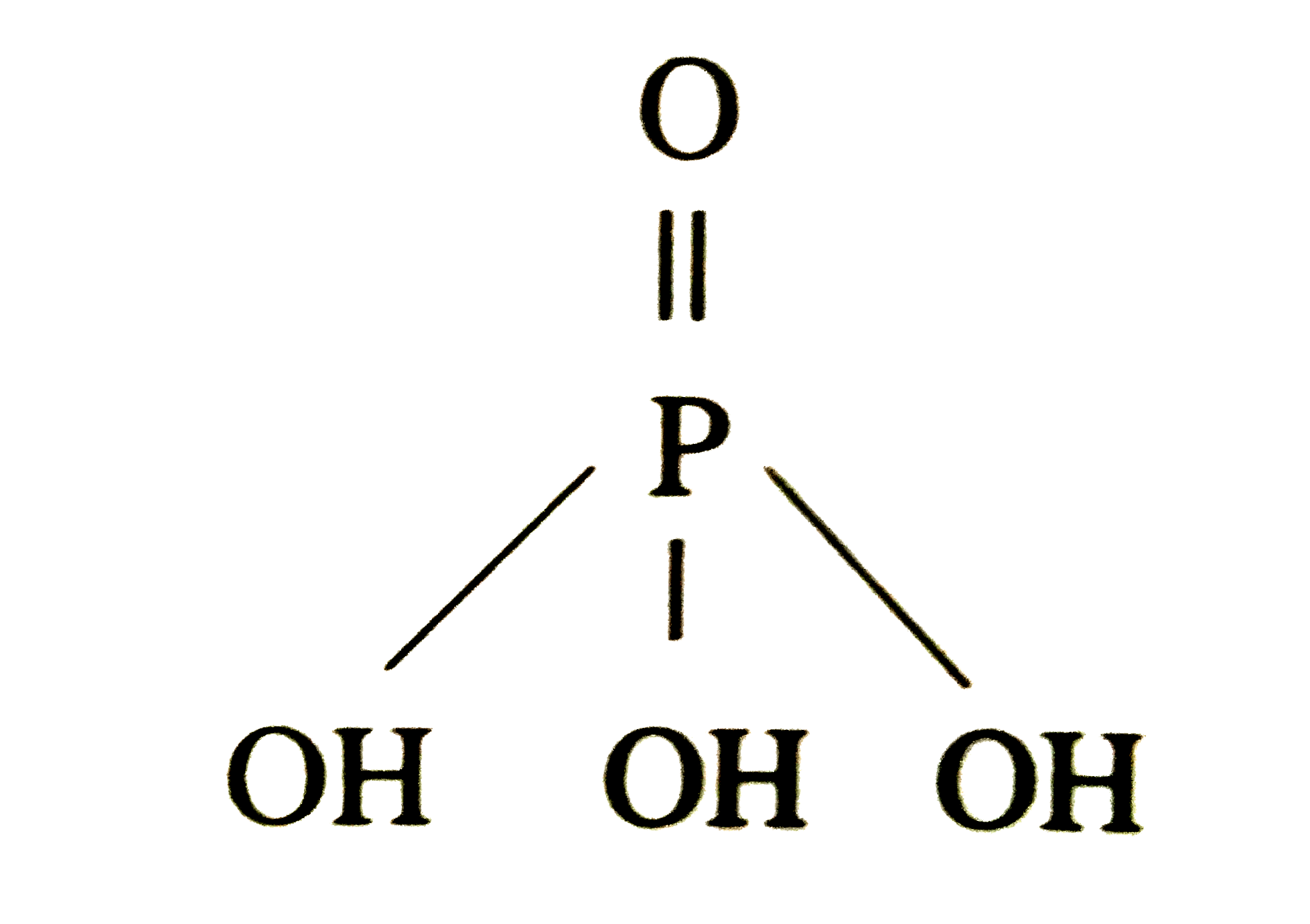 ( b) Pyrophosphoric Acid `(H_(4)P_(2)O_(7)`) : `HO-overset(OH)overset("|")underset("O")underset("||")("P ")-O-overset(OH)overset("|")underset("O")underset("||")("P ")-OH` |
|
Discussion
No Comment Found
Related InterviewSolutions
- Which of the following compounds is not cleaved by HI even at 525 K ?
- To a 25 mL H_(2)O_(2) solution excess of an acidified solution of potassium iodide was added. The iodine liberated required 20 " mL of " 0.3 N sodium thiosulphate solution Calculate the volume strength of H_(2)O_(2) solution.
- The suggested mechanism of a reaction is : (a) A+BhArrD("fast) "(b)A+Drarr2C("slow")Write the balanced equation of the reaction if its experimentally deduced rate equation is , rate k=[A]^(2)[B] Find the intermediate formed during the course of the reaction . Does the predicted rate law from the mechanism match the experimental rate law ?
- Which of these changes with time for a first-order reaction A Rate of reaction B . Rate constant C . Half-life
- What is the hybridisation of central atom in the product obtained along with hydrofluoric acid when complete hydrolysis of Xenon Hexa Fluoride takes place ?
- Which of the following amino acid forms sulphide bond in polypeptide
- Which of following pair is Diastereomers:
- What is the major product of the following reaction CH_3C-=C-CH_2-CH_3overset("1 mole of " Cl_2)to
- Which polymer is used in petrol tank linings ?
- Which of the following carbohydrates are branched polymer of glucose ?