Saved Bookmarks
| 1. |
Derive an equation for solution which shows relation between total pressure and mole fraction of volatile solute and volatile solvent and explain it by plotting graph. |
|
Answer» Solution :Suppose in a binary meaning volatile solution component 1 and component 2 is present. Their mole fraction is `X_(1)` and `X_(2)` and their vapour pressure is `p_(1)` and `p_(2)` respective. According to the Raoult.s law, a solution of volatile liquids, the PARTIAL vapour pressure of each component of the solution is directl proportional to its mole fraction present in solution. Thus, for componnent 1 , `p_(1)prop X_(1)` and `p_(1)=p_(1)^(0). X_(1)` where `p_(1)` is the vapour pressure of pure component 1 at the same temperature. Similarly, for component 2, `p_(2) prop X_(2)`and`p_(2)=p_(2)^(0).X_(2)`, where `p_(2)^(0)` represents the vapour pressure of the pure component 2. According to Dalton.s law of partial pressures, the total pressure (total p) over the solution phase in the container will be the sum of the partial pressures of the components of the solution. `p_("total")=p_(1)+p_(2)` `p_("total")=p_(1)^(0).x_(1)+p_(2)^(0).x_(2)` `=(1-x_(2))p_(1)^(0)+x_(2)p_(2)^(0)` `p_("total")=p_(1)^(0)+x_(2)(p_(2)^(0)-p_(1)^(0))` Following conclusions can be drawn from equation : (i) Total vapour pressure over the solution can be related to the mole fraction of any one component. (ii) Total vapour pressure over the solution VARIES linearly with the mole fraction of component 2. (iii)Depending on the vapour pressures of the pure components 1 and 2, total vapour pressure over the solution decreases or increases with the increase of the mole fraction of component 1. A plot of `p_(1)` or `p_(2)` versus the mole fractions `X_(1)` and `X_(2)` for a solution given a linear plot as shown in figure. 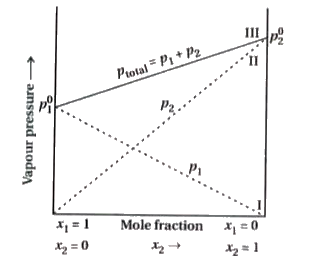 These lines (I and II) pass through the points for which `X_(1)` and `X_(2)` are EQUAL to unity. Similarly the plot (line III) of `p_("total")` versus `X_(2)` is also linear. The minimum value of `p_("total")` is `p_(1)^(0)` and the maximum value is `p_(2)^(0)`, assuming that component 1 is less volatile than component 2, i.e., `p_(1)^(0)lt p_(2)^(0)`. The COMPOSITION of vapour phase in equilibrium with the solution is determined by the partial presures of the components. If `Y_(1)` and `Y_(2)` are the mole fractions of the components 1 and 2 respectively in the vapour phase than, using Dalton.s law of partial pressures: `p_(1)=Y_(1)p_("total")` and `p_(2)=Y_(2)p_("total")` So, in general `p_(i)=Y_(i)p_("total")` |
|
Discussion
No Comment Found
Related InterviewSolutions
- Which of the following compounds is not cleaved by HI even at 525 K ?
- To a 25 mL H_(2)O_(2) solution excess of an acidified solution of potassium iodide was added. The iodine liberated required 20 " mL of " 0.3 N sodium thiosulphate solution Calculate the volume strength of H_(2)O_(2) solution.
- The suggested mechanism of a reaction is : (a) A+BhArrD("fast) "(b)A+Drarr2C("slow")Write the balanced equation of the reaction if its experimentally deduced rate equation is , rate k=[A]^(2)[B] Find the intermediate formed during the course of the reaction . Does the predicted rate law from the mechanism match the experimental rate law ?
- Which of these changes with time for a first-order reaction A Rate of reaction B . Rate constant C . Half-life
- What is the hybridisation of central atom in the product obtained along with hydrofluoric acid when complete hydrolysis of Xenon Hexa Fluoride takes place ?
- Which of the following amino acid forms sulphide bond in polypeptide
- Which of following pair is Diastereomers:
- What is the major product of the following reaction CH_3C-=C-CH_2-CH_3overset("1 mole of " Cl_2)to
- Which polymer is used in petrol tank linings ?
- Which of the following carbohydrates are branched polymer of glucose ?