Saved Bookmarks
| 1. |
Describe Ostwald process for the manufacture of Nitric acid. Give uses. |
|
Answer» Solution :THEORY: (i) `NH_3`is oxidised to NO by air or `O_2`at 1070 K in the presence of Pt or Rh - Pd alloy `4NH_3 + 5O_2 to 6H_2O +4NO` (ii) Nitric oxide formed is cooled and allowed to come into contact with oxygen or air when it is oxidised to nitrogen dioxide. `2NO + O_2 to 2NO_2` (iii) The nitrogen dioxide formed is dissolved in WATER in the presence of air or oxygen when nitric acid is obtained. `4NO_2 + 2H_2O + O_2 to 4NHO_3` Working of the Plant. The outline of the plant and its working are shown in Fig. 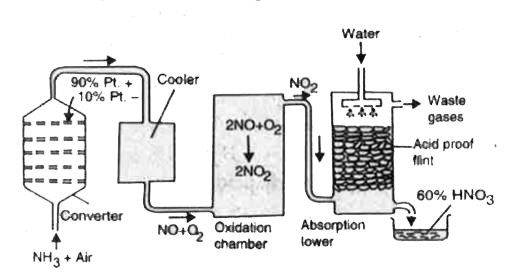 (i) Oxidation of ammonia. Ammonia prepared by Haber.s Process is MIXED with 10 times its volume of pure, dust, free, dry air. The mixture is passed through a converter made of either steel or aluminium with platinum gauze placed in it. The temperature of the converter is kept at about 1070 K. In the course of reaction, the catalyst platinum gauze gets a deposit of dust on it. Thus its efficiency is reduced. These days a more efficient catalyst consisting of an alloy of 90 percent platinum and 10 percent rhodium is being used. Here the ammonia is oxidised to nitric oxide. `4NH_3 + 5O_2 to 6H_2O + 4NO + `heat The reaction is exothermic. Therefore HEATING of the converter is required initially. Later on, the heat of reaction maintains the required temperature and no external heating is required. (ii) Oxidation of nitric oxide. The nitric oxide coming out of the converter, is cooled and passed through a chamber where it mixes with oxygen and oxidised to nitrogen dioxide. It is called oxidising chamber. `2NO + O_2 to 2NO_2` (iii) Absorption of nitrogen peroxide. The nitrogen dioxide from oxidation chamber is absorbed in water in the presence of oxygen. `2H_2O + 4NO_2 + O_2 to 2HNO_3` This solution of `HNO_3`is concentrated further. Uses of Nitric acid (i) Nitric acid finds extensive APPLICATION in the formation of fertilizers such as calcium nitrate `[CaO.Ca(NO)_2].` (ii) It has been used in the manufacture of sulphuric acid by Lead Chamber Process. (iii) It has been used for the preparation of perfumes, dyes, medicines etc. (iv) It finds application for the purification of silver and gold in the form of aqua regia. (v) It has been used for the manufacture of explosive substances like TNT, nitroglycerine, gun cotton, picric acid etc. in combination with concentrated sulphuric acid. (vi) Artificial silk has also been manufactured with its use. (vii) It is a very important laboratory reagent. |
|
Discussion
No Comment Found
Related InterviewSolutions
- Which of the following compounds is not cleaved by HI even at 525 K ?
- To a 25 mL H_(2)O_(2) solution excess of an acidified solution of potassium iodide was added. The iodine liberated required 20 " mL of " 0.3 N sodium thiosulphate solution Calculate the volume strength of H_(2)O_(2) solution.
- The suggested mechanism of a reaction is : (a) A+BhArrD("fast) "(b)A+Drarr2C("slow")Write the balanced equation of the reaction if its experimentally deduced rate equation is , rate k=[A]^(2)[B] Find the intermediate formed during the course of the reaction . Does the predicted rate law from the mechanism match the experimental rate law ?
- Which of these changes with time for a first-order reaction A Rate of reaction B . Rate constant C . Half-life
- What is the hybridisation of central atom in the product obtained along with hydrofluoric acid when complete hydrolysis of Xenon Hexa Fluoride takes place ?
- Which of the following amino acid forms sulphide bond in polypeptide
- Which of following pair is Diastereomers:
- What is the major product of the following reaction CH_3C-=C-CH_2-CH_3overset("1 mole of " Cl_2)to
- Which polymer is used in petrol tank linings ?
- Which of the following carbohydrates are branched polymer of glucose ?