Saved Bookmarks
| 1. |
Describe the construction and working of hydrogen -oxygen (H_(2) - O_(2)) fuel cell. |
|
Answer» Solution :(A) Principle : (1) The functioning of the fuel cell is based on the fact that the combustion reaction like `2H_(2(g)) + O_(2(g)) RARR 2H_(2)O_((g))` is EXOTHERMIC redox reaction and can be used to produce electricity . (2) The reactants of this fuel cell can be continuously supplied from outside, hence this type of galvanic cell can be used to supply electrical energy for a verylong period. 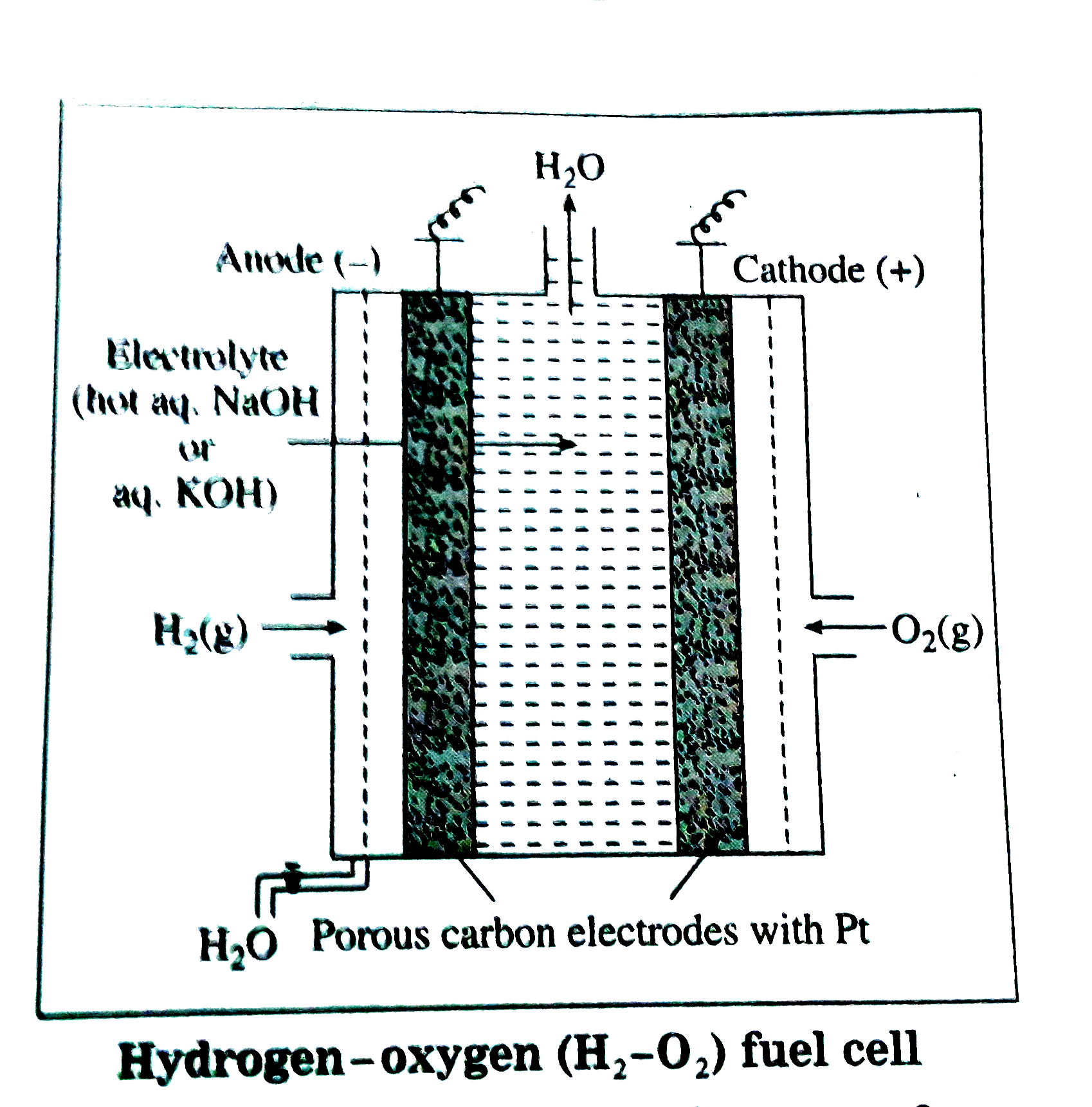 (B) Construction : (1) The fuel cell has porous electrodes with suitable catalyst since combustion of hydrogen is a slow reaction. (2) Anode and cathode cosistsof porous carbon rods impregnatedwith FINELY divided platinum which acts as a catalyst for the reactions. (3) The electrolyte used is not aqueous KOH solution in wich porous anode and cathode carbon rods are immersed. (4) `H_(2)` is continuously bubbled through anode while `O_(2)` gas is bubbled through cathode. (C ) Working (cell reactions) : (1) Oxidation at anode : `2H_(2(g)) + 4OH_((aq))^(-) rarr 4H_(2)O_((l)) + 4e^(-)` (oxidation half reaction) (2) Reduction at cathode : `O_(2(g)) + 2H_(2)O_((l)) + 4e^(-) rarr 4OH_((aq))^(-)` (reduction half reaction) (3) Net cell reaction : Additionof both the above reactions at anode and cathode gives a net cell reaction. `2H_(2(g)) + O_(2(g)) rarr 2H_(2)O_((l))` (overall cell reaction) (D) Representation of the cell : `""^(-)Pt|H_(2(g)) |underset((hot))(NaOH_((aq)))|O_(2(g))|Pt^(+)` `E_("cell")^(0) = E_("cathode")^(0) - E_("anode")^(0)` `= 0 .4- (-0.83)` `= 1.23 V` (E) Advantages : (1) The cell reactions do not cause any pollution. (2) The efficiency of this galvanic cell is the highest about `70%` as compared to ordinarygalvaniccells. (F) Drawbacks of `H_(2)l-O_(2)` fuel cell : (1) The cell requires expensive electrodes like `Pt, Pd`. (2) In practice, voltage is less than `1.23` voltdue to spontaneous reactions at the electrodes. (3) `H_(2)` gas is expensive and hazardous . (G) Applications : (1) It was successfully used in spacecraft. (2) It has potential applications in automobiles power generators for domestic and industrial uses. |
|
Discussion
No Comment Found
Related InterviewSolutions
- Which of the following compounds is not cleaved by HI even at 525 K ?
- To a 25 mL H_(2)O_(2) solution excess of an acidified solution of potassium iodide was added. The iodine liberated required 20 " mL of " 0.3 N sodium thiosulphate solution Calculate the volume strength of H_(2)O_(2) solution.
- The suggested mechanism of a reaction is : (a) A+BhArrD("fast) "(b)A+Drarr2C("slow")Write the balanced equation of the reaction if its experimentally deduced rate equation is , rate k=[A]^(2)[B] Find the intermediate formed during the course of the reaction . Does the predicted rate law from the mechanism match the experimental rate law ?
- Which of these changes with time for a first-order reaction A Rate of reaction B . Rate constant C . Half-life
- What is the hybridisation of central atom in the product obtained along with hydrofluoric acid when complete hydrolysis of Xenon Hexa Fluoride takes place ?
- Which of the following amino acid forms sulphide bond in polypeptide
- Which of following pair is Diastereomers:
- What is the major product of the following reaction CH_3C-=C-CH_2-CH_3overset("1 mole of " Cl_2)to
- Which polymer is used in petrol tank linings ?
- Which of the following carbohydrates are branched polymer of glucose ?