Saved Bookmarks
| 1. |
Describe the laboratory method of preparation of hydrogen gas. |
|
Answer» Solution :Laboratory method of preparation Principle: All the metals present above HYDROGEN in the reactivity series on reaction with dilute HCI or dilute `H_(2)SO_(4)` LIBERATE hydrogen gas. Zinc metal is preferred for this reaction. `Zn+2HCItoZnCI_(2)+H_(2)uarr` `Zn+H_(2)SO_(4)toZnSO_(4)+H_(4)uarr` 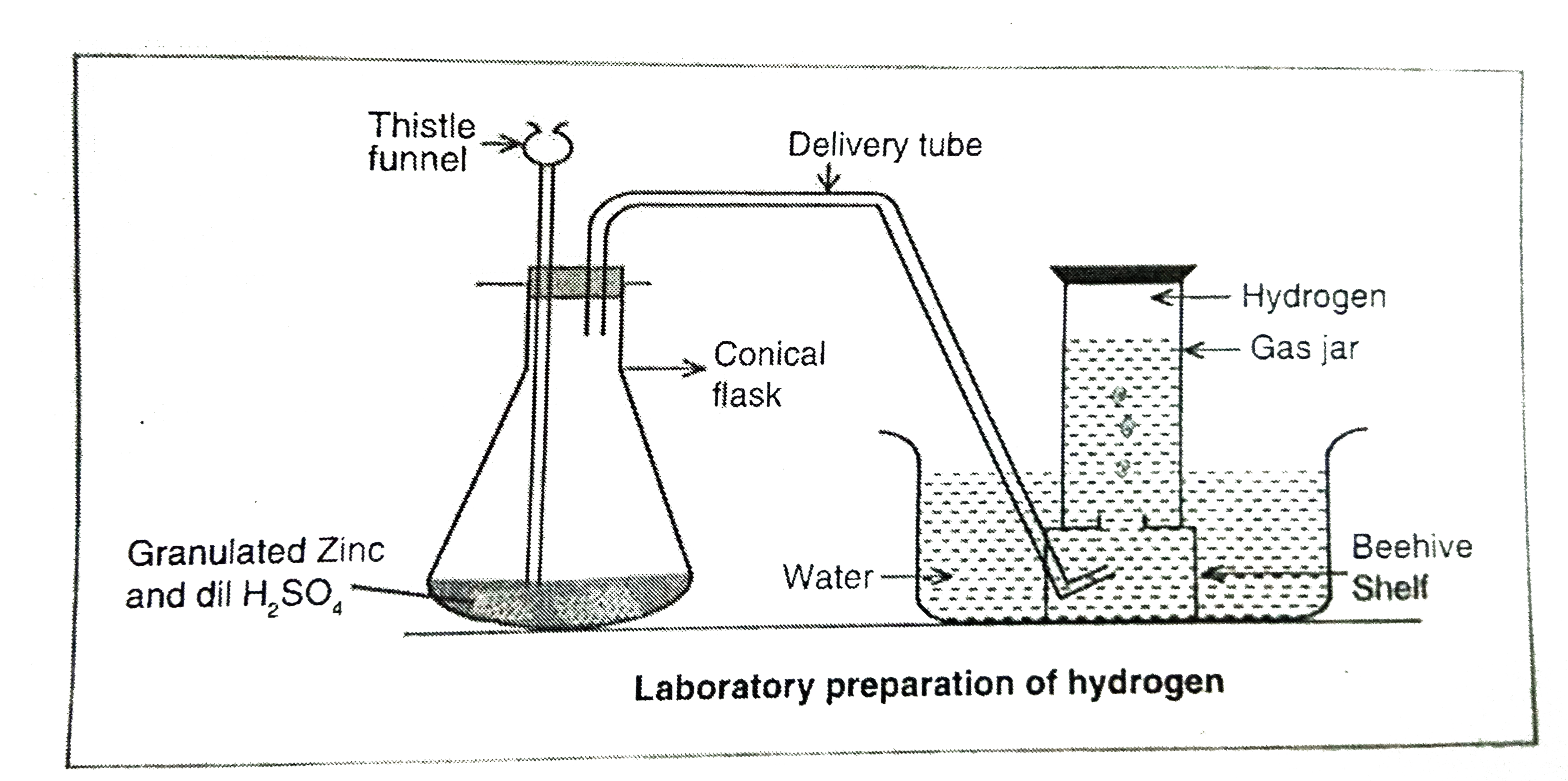 Experiment :Some granulated zinc pieces are taken in a flat-BOTTOM flask bottom flask fitted with a two holed air tigth cork. In one hole, a thistle a thistle funnel is inerted and in anoter hole, a long dilivery tube is inseted. Dilute `H_(2)SO_(4)` is added to the flask drop-wise through the thistile funnel. Observation: Effervescence is OBSERVED in the initial stages of the reaction. Thena colourless, and odourless gas evolves. Collection of gas: Hydrogen is collected by the downward DISPLACEMENT of water. |
|
Discussion
No Comment Found
Related InterviewSolutions
- Which of the following phenomenon is an effect acid rain ?
- Which of the following can be used to reduce suspended particulate matter in atmosphere in mine areas ?
- What are noble gases ? Mention their uses.
- What will be the formula of the sulphate and sulphite of a trivalent metal that isM^(+3) ?
- Which among the following is an element ?
- Xenon and krypton is used in electric bulbs to slow down the sublimation of tungsten.
- Whichof the following substances does not form a curdy precipitate when it is added to hard water ?
- Why are droplets of water observed on the walls of a glass tumbler containing ice ?
- What are homogeneous and heterogeneous mixtures ? Give one example for each.
- Which among the following is true regarding aqueous solution of sulphur trioxide and sodium oxide?